Involvement of the ERK pathway in the protective effects of glycyrrhizic acid against the MPP+-induced apoptosis of dopaminergic neuronal cells
- PMID: 24993693
- PMCID: PMC4121344
- DOI: 10.3892/ijmm.2014.1830
Involvement of the ERK pathway in the protective effects of glycyrrhizic acid against the MPP+-induced apoptosis of dopaminergic neuronal cells
Abstract
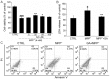
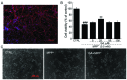
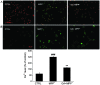
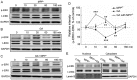
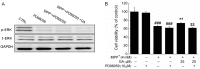
Glycyrrhizic acid (GA), a major compound separated from Radix Glycyrrhizae, has been shwon to exert various biochemical effects, including neuroprotective effects. In the present study, we investigated the protective effects of GA against 1-methyl-4-phenylpyridinium (MPP+)‑induced damage to differentiated PC12 (DPC12) cells. Compared with the MPP+-treated cells, GA markedly improved cell viability, restored mitochondrial dysfunction, suppressed the overexpression of cleaved poly(ADP-ribose) polymerase (PARP), and suppressed the overproduction of lactate dehydrogenase (LDH) and intracellular Ca2+ overload. The protective effects of GA on cell survival were further confirmed in primary cortical neurons. GA markedly increased the expression of phosphorylated extracellular signal-regulated kinase (p-ERK), as well as its migration from the cytoplasm to nucleus. PD98059, an inhibitor of ERK, blocked GA-enhanced ERK activation and reduced cell viability. However, pre-treatment with GA had no effects on the expression of phosphorylated AKT (p-AKT) and total AKT (t-AKT). These results indicate that the GA-mediated neuroprotective effects are associated with its modulation of multiple anti-apoptotic and pro-apoptotic factors, particularly the ERK signaling pathway. This study provides evidence supporting the use of GA as a potential therapeutic agent for the treatment of neurodegenerative diseases and neuronal injury.
Figures


Similar articles
-
Paeoniflorin, a natural neuroprotective agent, modulates multiple anti-apoptotic and pro-apoptotic pathways in differentiated PC12 cells.Cell Mol Neurobiol. 2013 May;33(4):521-9. doi: 10.1007/s10571-013-9914-y. Epub 2013 Feb 24. Cell Mol Neurobiol. 2013. PMID: 23436209 Free PMC article.
-
ERKs and mitochondria-related pathways are essential for glycyrrhizic acid-mediated neuroprotection against glutamate-induced toxicity in differentiated PC12 cells.Braz J Med Biol Res. 2014 Sep;47(9):773-9. doi: 10.1590/1414-431x20143760. Epub 2014 Jul 25. Braz J Med Biol Res. 2014. PMID: 25075574 Free PMC article.
-
PI3-K/Akt and ERK pathways activated by VEGF play opposite roles in MPP+-induced neuronal apoptosis.Neurochem Int. 2011 Nov;59(6):945-53. doi: 10.1016/j.neuint.2011.07.005. Epub 2011 Jul 12. Neurochem Int. 2011. PMID: 21781996
-
Secalonic acid A protects dopaminergic neurons from 1-methyl-4-phenylpyridinium (MPP⁺)-induced cell death via the mitochondrial apoptotic pathway.Eur J Pharmacol. 2013 Aug 5;713(1-3):58-67. doi: 10.1016/j.ejphar.2013.04.029. Epub 2013 May 9. Eur J Pharmacol. 2013. PMID: 23665112
-
Neuroprotective effects of glycyrrhizic acid and 18beta-glycyrrhetinic acid in PC12 cells via modulation of the PI3K/Akt pathway.J Agric Food Chem. 2009 Jan 28;57(2):754-61. doi: 10.1021/jf802864k. J Agric Food Chem. 2009. PMID: 19105645
Cited by
-
Glycyrrhizic Acid Mitigates Haloperidol-Induced Neurotoxicity in SHSY-5Y Cells and Rats Via Activation of PI3k/Akt/Nrf2 Pathways.Neurochem Res. 2025 Jan 9;50(1):75. doi: 10.1007/s11064-024-04319-1. Neurochem Res. 2025. PMID: 39786594
-
Orexin B protects dopaminergic neurons from 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity associated with reduced extracellular signal-regulated kinase phosphorylation.Mol Biol Rep. 2024 May 24;51(1):669. doi: 10.1007/s11033-024-09587-2. Mol Biol Rep. 2024. PMID: 38787465
-
Neuroprotective Effects of the DPP4 Inhibitor Vildagliptin in In Vivo and In Vitro Models of Parkinson's Disease.Int J Mol Sci. 2022 Feb 21;23(4):2388. doi: 10.3390/ijms23042388. Int J Mol Sci. 2022. PMID: 35216503 Free PMC article.
-
The mTOR inhibition in concurrence with ERK1/2 activation is involved in excessive autophagy induced by glycyrrhizin in hepatocellular carcinoma.Cancer Med. 2017 Aug;6(8):1941-1951. doi: 10.1002/cam4.1127. Epub 2017 Jul 3. Cancer Med. 2017. PMID: 28675698 Free PMC article.
-
Therapeutic potential of thymoquinone and its nanoformulations in neuropsychological disorders: a comprehensive review on molecular mechanisms in preclinical studies.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jun;397(6):3541-3564. doi: 10.1007/s00210-023-02832-8. Epub 2023 Nov 27. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38010395 Review.
References
-
- Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–272. - PubMed
-
- Surendran S, Rajasankar S. Parkinson’s disease: oxidative stress and therapeutic approaches. Neurol Sci. 2010;31:531–540. - PubMed
-
- Schulz JB, Falkenburger BH. Neuronal pathology in Parkinson’s disease. Cell Tissue Res. 2004;318:135–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

