5'-AMP-activated protein kinase (AMPK) supports the growth of aggressive experimental human breast cancer tumors
- PMID: 24993821
- PMCID: PMC4132788
- DOI: 10.1074/jbc.M114.576371
5'-AMP-activated protein kinase (AMPK) supports the growth of aggressive experimental human breast cancer tumors
Abstract
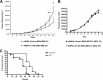
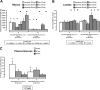
Rapid tumor growth can establish metabolically stressed microenvironments that activate 5'-AMP-activated protein kinase (AMPK), a ubiquitous regulator of ATP homeostasis. Previously, we investigated the importance of AMPK for the growth of experimental tumors prepared from HRAS-transformed mouse embryo fibroblasts and for primary brain tumor development in a rat model of neurocarcinogenesis. Here, we used triple-negative human breast cancer cells in which AMPK activity had been knocked down to investigate the contribution of AMPK to experimental tumor growth and core glucose metabolism. We found that AMPK supports the growth of fast-growing orthotopic tumors prepared from MDA-MB-231 and DU4475 breast cancer cells but had no effect on the proliferation or survival of these cells in culture. We used in vitro and in vivo metabolic profiling with [(13)C]glucose tracers to investigate the contribution of AMPK to core glucose metabolism in MDA-MB-231 cells, which have a Warburg metabolic phenotype; these experiments indicated that AMPK supports tumor glucose metabolism in part through positive regulation of glycolysis and the nonoxidative pentose phosphate cycle. We also found that AMPK activity in the MDA-MB-231 tumors could systemically perturb glucose homeostasis in sensitive normal tissues (liver and pancreas). Overall, our findings suggest that the contribution of AMPK to the growth of aggressive experimental tumors has a critical microenvironmental component that involves specific regulation of core glucose metabolism.
Keywords: AMP-activated Kinase (AMPK); Glucose Metabolism; Metabolic Profiling; Triple Negative; Tumor Metabolism; Tumor Microenvironment; Warburg Effect.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

