Vessel formation. De novo formation of a distinct coronary vascular population in neonatal heart
- PMID: 24994653
- PMCID: PMC4275002
- DOI: 10.1126/science.1251487
Vessel formation. De novo formation of a distinct coronary vascular population in neonatal heart
Abstract
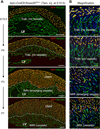
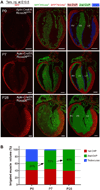
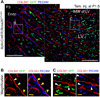
The postnatal coronary vessels have been viewed as developing through expansion of vessels formed during the fetal period. Using genetic lineage tracing, we found that a substantial portion of postnatal coronary vessels arise de novo in the neonatal mouse heart, rather than expanding from preexisting embryonic vasculature. Our data show that lineage conversion of neonatal endocardial cells during trabecular compaction generates a distinct compartment of the coronary circulation located within the inner half of the ventricular wall. This lineage conversion occurs within a brief period after birth and provides an efficient means of rapidly augmenting the coronary vasculature. This mechanism of postnatal coronary vascular growth provides avenues for understanding and stimulating cardiovascular regeneration following injury and disease.
Copyright © 2014, American Association for the Advancement of Science.
Figures

Comment in
-
Development. A crowning achievement for deciphering coronary origins.Science. 2014 Jul 4;345(6192):28-9. doi: 10.1126/science.1256866. Science. 2014. PMID: 24994633 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

