Three-dimensional osteogenic and chondrogenic systems to model osteochondral physiology and degenerative joint diseases
- PMID: 24994814
- PMCID: PMC5413932
- DOI: 10.1177/1535370214539232
Three-dimensional osteogenic and chondrogenic systems to model osteochondral physiology and degenerative joint diseases
Abstract
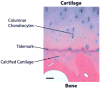
Tissue engineered constructs have the potential to function as in vitro pre-clinical models of normal tissue function and disease pathogenesis for drug screening and toxicity assessment. Effective high throughput assays demand minimal systems with clearly defined performance parameters. These systems must accurately model the structure and function of the human organs and their physiological response to different stimuli. Musculoskeletal tissues present unique challenges in this respect, as they are load-bearing, matrix-rich tissues whose functionality is intimately connected to the extracellular matrix and its organization. Of particular clinical importance is the osteochondral junction, the target tissue affected in degenerative joint diseases, such as osteoarthritis (OA), which consists of hyaline articular cartilage in close interaction with subchondral bone. In this review, we present an overview of currently available in vitro three-dimensional systems for bone and cartilage tissue engineering that mimic native physiology, and the utility and limitations of these systems. Specifically, we address the need to combine bone, cartilage and other tissues to form an interactive microphysiological system (MPS) to fully capture the biological complexity and mechanical functions of the osteochondral junction of the articular joint. The potential applications of three-dimensional MPSs for musculoskeletal biology and medicine are highlighted.
Keywords: Tissue engineering; biomaterial scaffold; bone; cartilage; drug screening; osteoarthritis.
© 2014 by the Society for Experimental Biology and Medicine.
Figures




References
-
- Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–9. - PubMed
-
- Hoemann CD, Lafantaisie-Favreau C-H, Lascau-Coman V, Chen G, Guzmán-Morales J. The cartilage-bone interface. J Knee Surg. 2012;25:85–97. - PubMed
-
- Kouri JB, Lavalle C. Do chondrocytes undergo “activation” and “transdifferentiation” during the pathogenesis of osteoarthritis? A review of the ultrastructural and immunohistochemical evidence. Histol Histopathol. 2006;21:793–802. - PubMed
-
- Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33:1–13. - PubMed
-
- Coates EE, Fisher JP. Phenotypic variations in chondrocyte subpopulations and their response to in vitro culture and external stimuli. Ann Biomed Eng. 2010;38:3371–88. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

