Opinion: uracil DNA glycosylase (UNG) plays distinct and non-canonical roles in somatic hypermutation and class switch recombination
- PMID: 24994819
- PMCID: PMC4184391
- DOI: 10.1093/intimm/dxu071
Opinion: uracil DNA glycosylase (UNG) plays distinct and non-canonical roles in somatic hypermutation and class switch recombination
Abstract
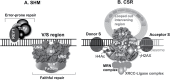
Activation-induced cytidine deaminase (AID) is essential to class switch recombination (CSR) and somatic hypermutation (SHM). Uracil DNA glycosylase (UNG), a member of the base excision repair complex, is required for CSR. The role of UNG in CSR and SHM is extremely controversial. AID deficiency in mice abolishes both CSR and SHM, while UNG-deficient mice have drastically reduced CSR but augmented SHM raising a possibility of differential functions of UNG in CSR and SHM. Interestingly, UNG has been associated with a CSR-specific repair adapter protein Brd4, which interacts with acetyl histone 4, γH2AX and 53BP1 to promote non-homologous end joining during CSR. A non-canonical scaffold function of UNG, but not the catalytic activity, can be attributed to the recruitment of essential repair proteins associated with the error-free repair during SHM, and the end joining during CSR.
Keywords: AID; Brd4; CSR; NHEJ; SHM; UNG.
© The Japanese Society for Immunology. 2014. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Shinkura R., Ito S., Begum N. A., et al. 2004. Separate domains of AID are required for somatic hypermutation and class-switch recombination. Nat. Immunol. 5:707. - PubMed
-
- Barreto V., Reina-San-Martin B., Ramiro A. R., McBride K. M., Nussenzweig M. C. 2003. C-terminal deletion of AID uncouples class switch recombination from somatic hypermutation and gene conversion. Mol. Cell 12:501. - PubMed
-
- Ta V. T., Nagaoka H., Catalan N., et al. 2003. AID mutant analyses indicate requirement for class-switch-specific cofactors. Nat. Immunol. 4:843. - PubMed
-
- Conticello S. G., Langlois M. A., Yang Z., Neuberger M. S. 2007. DNA deamination in immunity: AID in the context of its APOBEC relatives. Adv. Immunol. 94:37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

