Contrast-enhanced time-resolved MRA for follow-up of intracranial aneurysms treated with the pipeline embolization device
- PMID: 24994828
- PMCID: PMC7965189
- DOI: 10.3174/ajnr.A4008
Contrast-enhanced time-resolved MRA for follow-up of intracranial aneurysms treated with the pipeline embolization device
Abstract
Background and purpose: Endovascular reconstruction and flow diversion by using the Pipeline Embolization Device is an effective treatment for complex cerebral aneurysms. Accurate noninvasive alternatives to DSA for follow-up after Pipeline Embolization Device treatment are desirable. This study evaluated the accuracy of contrast-enhanced time-resolved MRA for this purpose, hypothesizing that contrast-enhanced time-resolved MRA will be comparable with DSA and superior to 3D-TOF MRA.
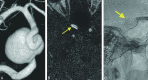
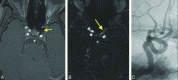
Materials and methods: During a 24-month period, 37 Pipeline Embolization Device-treated intracranial aneurysms in 26 patients underwent initial follow-up by using 3D-TOF MRA, contrast-enhanced time-resolved MRA, and DSA. MRA was performed on a 1.5T unit by using 3D-TOF and time-resolved imaging of contrast kinetics. All patients underwent DSA a median of 0 days (range, 0-68) after MRA. Studies were evaluated for aneurysm occlusion, quality of visualization of the reconstructed artery, and measurable luminal diameter of the Pipeline Embolization Device, with DSA used as the reference standard.
Results: The sensitivity, specificity, and positive and negative predictive values of contrast-enhanced time-resolved MRA relative to DSA for posttreatment aneurysm occlusion were 96%, 85%, 92%, and 92%. Contrast-enhanced time-resolved MRA demonstrated superior quality of visualization (P = .0001) and a higher measurable luminal diameter (P = .0001) of the reconstructed artery compared with 3D-TOF MRA but no significant difference compared with DSA. Contrast-enhanced time-resolved MRA underestimated the luminal diameter of the reconstructed artery by 0.965 ± 0.497 mm (27% ± 13%) relative to DSA.
Conclusions: Contrast-enhanced time-resolved MRA is a reliable noninvasive method for monitoring intracranial aneurysms following flow diversion and vessel reconstruction by using the Pipeline Embolization Device.
© 2014 by American Journal of Neuroradiology.
Figures

References
-
- Molyneux AJ, Kerr RSC, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol 2009;8:427–33 - PMC - PubMed
-
- Fiorella D, Kelly ME, Turner R, et al. Endovascular treatment of cerebral aneurysms: current devices, emerging therapies and future technology for the management of cerebral aneurysms. Endovasc Today 2008;7:53–65
-
- Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013;267:858–68 - PubMed
-
- Delgado Almandoz JE, Crandall BM, Fease JL, et al. Successful endovascular treatment of three fusiform cerebral aneurysms with the Pipeline embolization device in a patient with dilating HIV vasculopathy. J Neurointerv Surg 2014;6:e12. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
