Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model
- PMID: 24994926
- PMCID: PMC4279738
- DOI: 10.1681/ASN.2013121312
Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model
Abstract
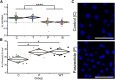
Autosomal dominant polycystic kidney disease (ADPKD) is a leading cause of ESRD. A central defect associated with ADPKD pathology is elevated levels of 3', 5'-cyclic AMP (cAMP). Compounds such as tolvaptan and pasireotide, which indirectly reduce adenylyl cyclase 6 (AC6) activity, have hence proven effective in slowing cyst progression. Here, we tested the efficacy of these compounds individually and in combination in a hypomorphic PKD1 model, Pkd1(R3277C/R3277C) (Pkd1(RC/RC)), in a 5-month preclinical trial. Initially, the Pkd1(RC/RC) model was inbred into the C57BL/6 background, minimizing disease variability, and the pathogenic effect of elevating cAMP was confirmed by treatment with the AC6 stimulant desmopressin. Treatment with tolvaptan or pasireotide alone markedly reduced cyst progression and in combination showed a clear additive effect. Furthermore, combination treatment significantly reduced cystic and fibrotic volume and decreased cAMP to wild-type levels. We also showed that Pkd1(RC/RC) mice experience hepatic hypertrophy that can be corrected by pasireotide. The observed additive effect reinforces the central role of AC6 and cAMP in ADPKD pathogenesis and highlights the likely benefit of combination therapy for patients with ADPKD.
Keywords: cyclic AMP; polycystic kidney disease; somatostatin; vasopressin.
Copyright © 2015 by the American Society of Nephrology.
Figures


References
-
- Harris PC, Torres VE: Polycystic kidney disease, autosomal dominant. In: GeneReviews, edited by Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, Seattle, University of Washington, 1993 [Updated 2011] - PubMed
-
- Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 - PubMed
-
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 - PubMed
-
- Kusano E, Murayama N, Werness JL, Christensen S, Homma S, Yusufi AN, Dousa TP: Effects of calcium on the vasopressin-sensitive cAMP metabolism in medullary tubules. Am J Physiol 249: F956–F966, 1985 - PubMed
-
- Kusano E, Yoshida I, Takeda S, Homma S, Yusufi AN, Dousa TP, Asano Y: Nephron distribution of total low Km cyclic AMP phosphodiesterase in mouse, rat and rabbit kidney. Tohoku J Exp Med 193: 207–220, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

