An EMMPRIN-γ-catenin-Nm23 complex drives ATP production and actomyosin contractility at endothelial junctions
- PMID: 24994937
- PMCID: PMC4150062
- DOI: 10.1242/jcs.149518
An EMMPRIN-γ-catenin-Nm23 complex drives ATP production and actomyosin contractility at endothelial junctions
Abstract
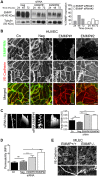
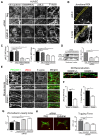
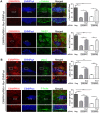
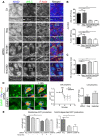
Cell-cell adhesions are important sites through which cells experience and resist forces. In endothelial cells, these forces regulate junction dynamics and determine endothelial barrier strength. We identify the Ig superfamily member EMMPRIN (also known as basigin) as a coordinator of forces at endothelial junctions. EMMPRIN localization at junctions correlates with endothelial junction strength in different mouse vascular beds. Accordingly, EMMPRIN-deficient mice show altered junctions and increased junction permeability. Lack of EMMPRIN alters the localization and function of VE-cadherin (also known as cadherin-5) by decreasing both actomyosin contractility and tugging forces at endothelial cell junctions. EMMPRIN ensures proper actomyosin-driven maturation of competent endothelial junctions by forming a molecular complex with γ-catenin (also known as junction plakoglobin) and Nm23 (also known as NME1), a nucleoside diphosphate kinase, thereby locally providing ATP to fuel the actomyosin machinery. These results provide a novel mechanism for the regulation of actomyosin contractility at endothelial junctions and might have broader implications in biological contexts such as angiogenesis, collective migration and tissue morphogenesis by coupling compartmentalized energy production to junction assembly.
Keywords: ATP; Actomyosin contractility; EMMPRIN; Endothelial junctions; NDPK; Nm23; Nucleoside diphosphate kinase; Vascular integrity; γ-catenin.
© 2014. Published by The Company of Biologists Ltd.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

