Toward a hierarchy of mechanisms in CaMKII-mediated arrhythmia
- PMID: 24994983
- PMCID: PMC4062880
- DOI: 10.3389/fphar.2014.00110
Toward a hierarchy of mechanisms in CaMKII-mediated arrhythmia
Abstract
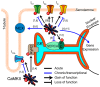
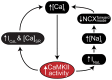
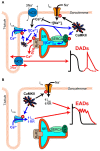
Calcium/calmodulin-dependent protein kinase II (CaMKII) activity has been shown to contribute to arrhythmogenesis in a remarkably broad range of cardiac pathologies. Several of these involve significant structural and electrophysiologic remodeling, whereas others are due to specific channelopathies, and are not typically associated with arrhythmogenic changes to protein expression or cellular and tissue structure. The ability of CaMKII to contribute to arrhythmia across such a broad range of phenotypes suggests one of two interpretations regarding the role of CaMKII in cardiac arrhythmia: (1) some CaMKII-dependent mechanism is a common driver of arrhythmia irrespective of the specific etiology of the disease, or (2) these different etiologies expose different mechanisms by which CaMKII is capable of promoting arrhythmia. In this review, we dissect the available mechanistic evidence to explore these two possibilities and discuss how the various molecular actions of CaMKII promote arrhythmia in different pathophysiologic contexts.
Keywords: CaMKII; afterdepolarizations; arrhythmias; cardiovascular diseases; ryanodine receptor.
Figures
References
-
- Aiba T., Barth A. S., Hesketh G. G., Hashambhoy Y. L., Chakir K., Tunin R. S., et al. (2013). Cardiac resynchronization therapy improves altered Na channel gating in canine model of dyssynchronous heart failure. Circ. Arrhythm. Electrophysiol. 6 546–554 10.1161/CIRCEP.113.000400 - DOI - PMC - PubMed
-
- Ashpole N. M., Herren A. W., Ginsburg K. S., Brogan J. D., Johnson D. E., Cummins T. R., et al. (2012). Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J. Biol. Chem. 287 19856–19869. 10.1074/jbc.M111.322537 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

