A modified controlled cortical impact technique to model mild traumatic brain injury mechanics in mice
- PMID: 24994996
- PMCID: PMC4061598
- DOI: 10.3389/fneur.2014.00100
A modified controlled cortical impact technique to model mild traumatic brain injury mechanics in mice
Abstract
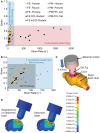
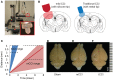
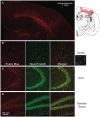
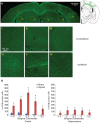
For the past 25 years, controlled cortical impact (CCI) has been a useful tool in traumatic brain injury (TBI) research, creating injury patterns that includes primary contusion, neuronal loss, and traumatic axonal damage. However, when CCI was first developed, very little was known on the underlying biomechanics of mild TBI. This paper uses information generated from recent computational models of mild TBI in humans to alter CCI and better reflect the biomechanical conditions of mild TBI. Using a finite element model of CCI in the mouse, we adjusted three primary features of CCI: the speed of the impact to achieve strain rates within the range associated with mild TBI, the shape, and material of the impounder to minimize strain concentrations in the brain, and the impact depth to control the peak deformation that occurred in the cortex and hippocampus. For these modified cortical impact conditions, we observed peak strains and strain rates throughout the brain were significantly reduced and consistent with estimated strains and strain rates observed in human mild TBI. We saw breakdown of the blood-brain barrier but no primary hemorrhage. Moreover, neuronal degeneration, axonal injury, and both astrocytic and microglia reactivity were observed up to 8 days after injury. Significant deficits in rotarod performance appeared early after injury, but we observed no impairment in spatial object recognition or contextual fear conditioning response 5 and 8 days after injury, respectively. Together, these data show that simulating the biomechanical conditions of mild TBI with a modified cortical impact technique produces regions of cellular reactivity and neuronal loss that coincide with only a transient behavioral impairment.
Keywords: biomechanics; controlled cortical impact; glia reactivity; mild traumatic brain injury; strain rate.
Figures
Similar articles
-
The Controlled Cortical Impact Model: Applications, Considerations for Researchers, and Future Directions.Front Neurol. 2016 Aug 17;7:134. doi: 10.3389/fneur.2016.00134. eCollection 2016. Front Neurol. 2016. PMID: 27582726 Free PMC article. Review.
-
A semicircular controlled cortical impact produces long-term motor and cognitive dysfunction that correlates well with damage to both the sensorimotor cortex and hippocampus.Brain Res. 2014 Aug 12;1576:18-26. doi: 10.1016/j.brainres.2014.05.042. Epub 2014 Jun 4. Brain Res. 2014. PMID: 24905625
-
Criteria to define mild, moderate, and severe traumatic brain injury in the mouse controlled cortical impact model.Exp Neurol. 2018 Dec;310:48-57. doi: 10.1016/j.expneurol.2018.07.004. Epub 2018 Jul 12. Exp Neurol. 2018. PMID: 30017882 Review.
-
A Machine Learning Approach to Investigate the Uncertainty of Tissue-Level Injury Metrics for Cerebral Contusion.Front Bioeng Biotechnol. 2021 Oct 8;9:714128. doi: 10.3389/fbioe.2021.714128. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34692652 Free PMC article.
-
Relationship of calpain-mediated proteolysis to the expression of axonal and synaptic plasticity markers following traumatic brain injury in mice.Exp Neurol. 2006 Sep;201(1):253-65. doi: 10.1016/j.expneurol.2006.04.013. Epub 2006 Jun 30. Exp Neurol. 2006. PMID: 16814284
Cited by
-
Assessing the Global Impact on the Mouse Kidney After Traumatic Brain Injury: A Transcriptomic Study.J Inflamm Res. 2022 Aug 24;15:4833-4851. doi: 10.2147/JIR.S375088. eCollection 2022. J Inflamm Res. 2022. PMID: 36042866 Free PMC article.
-
Pharmacokinetics and efficacy of PT302, a sustained-release Exenatide formulation, in a murine model of mild traumatic brain injury.Neurobiol Dis. 2019 Apr;124:439-453. doi: 10.1016/j.nbd.2018.11.023. Epub 2018 Nov 22. Neurobiol Dis. 2019. PMID: 30471415 Free PMC article.
-
Wide-field calcium imaging reveals widespread changes in cortical functional connectivity following mild traumatic brain injury in the mouse.Neurobiol Dis. 2023 Jan;176:105943. doi: 10.1016/j.nbd.2022.105943. Epub 2022 Dec 5. Neurobiol Dis. 2023. PMID: 36476979 Free PMC article.
-
Transplanted interneurons improve memory precision after traumatic brain injury.Nat Commun. 2019 Nov 14;10(1):5156. doi: 10.1038/s41467-019-13170-w. Nat Commun. 2019. PMID: 31727894 Free PMC article.
-
Cypin: A novel target for traumatic brain injury.Neurobiol Dis. 2018 Nov;119:13-25. doi: 10.1016/j.nbd.2018.07.019. Epub 2018 Jul 19. Neurobiol Dis. 2018. PMID: 30031156 Free PMC article.
References
-
- National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Centers for Disease Control and Prevention; (2003).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

