Expression pattern of thyroid hormone transporters in the postnatal mouse brain
- PMID: 24994998
- PMCID: PMC4061481
- DOI: 10.3389/fendo.2014.00092
Expression pattern of thyroid hormone transporters in the postnatal mouse brain
Abstract
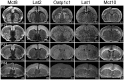
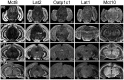
For a comprehensive description of the tissue-specific thyroidal state under normal as well as under pathophysiological conditions it is of utmost importance to include thyroid hormone (TH) transporters in the analysis as well. The current knowledge of the cell-specific repertoire of TH transporters, however, is still rather limited, although several TH transporting proteins have been identified. Here, we describe the temporal and spatial distribution pattern of the most prominent TH transporters in the postnatal mouse brain. For that purpose, we performed radioactive in situ hybridization studies in order to analyze the cellular mRNA expression pattern of the monocarboxylate transporters Mct8 and Mct10, the L-type amino acid transporters Lat1 and Lat2 as well as the organic anion transporting peptide Oatp1c1 at different postnatal time points. Highest TH transporter expression levels in the CNS were observed at postnatal day 6 and 12, while hybridization signal intensities visibly declined after the second postnatal week. The only exception was Mct10 for which the strongest signals could be observed in white matter regions at postnatal day 21 indicating that this transporter is preferentially expressed in mature oligodendrocytes. Whereas Mct8 and Lat2 showed an overlapping neuronal mRNA expression pattern in the cerebral cortex, hippocampus, and in the hypothalamus, Oatp1c1 and Lat1 specific signals were most prominent in capillary endothelial cells throughout the CNS. In the choroid plexus, expression of three transporters (Mct8, Lat2, and Oatp1c1) could be detected, whereas in other brain areas (e.g., striatum, thalamus, and brain stem nuclei) only one of the transporter candidates appeared to be present. Overall, our study revealed a distinct mRNA distribution pattern for each of the TH transporter candidates. Further studies will reveal to which extent these transporters contribute to the cell-specific TH uptake and efflux in the mouse CNS.
Keywords: CNS; Lat1; Lat2; Mct10; Mct8; Oatp1c1; T3; T4.
Figures


Similar articles
-
Mosaic Expression of Thyroid Hormone Regulatory Genes Defines Cell Type-Specific Dependency in the Developing Chicken Cerebellum.Cerebellum. 2016 Dec;15(6):710-725. doi: 10.1007/s12311-015-0744-y. Cerebellum. 2016. PMID: 26559893
-
Characterization of Chicken Thyroid Hormone Transporters.Endocrinology. 2016 Jun;157(6):2560-74. doi: 10.1210/en.2015-2025. Epub 2016 Apr 12. Endocrinology. 2016. PMID: 27070099
-
The expression of thyroid hormone transporters in the human fetal cerebral cortex during early development and in N-Tera-2 neurodifferentiation.J Physiol. 2011 Jun 1;589(Pt 11):2827-45. doi: 10.1113/jphysiol.2011.207290. Epub 2011 Mar 21. J Physiol. 2011. PMID: 21486766 Free PMC article.
-
The pathophysiological consequences of thyroid hormone transporter deficiencies: Insights from mouse models.Biochim Biophys Acta. 2013 Jul;1830(7):3974-8. doi: 10.1016/j.bbagen.2012.04.009. Epub 2012 Apr 20. Biochim Biophys Acta. 2013. PMID: 22543196 Review.
-
Molecular Mechanisms of Thyroid Hormone Transport by l-Type Amino Acid Transporter.Exp Clin Endocrinol Diabetes. 2020 Jun;128(6-07):379-382. doi: 10.1055/a-1032-8369. Epub 2019 Nov 18. Exp Clin Endocrinol Diabetes. 2020. PMID: 31739345 Review.
Cited by
-
Transport, Metabolism, and Function of Thyroid Hormones in the Developing Mammalian Brain.Front Endocrinol (Lausanne). 2019 Apr 3;10:209. doi: 10.3389/fendo.2019.00209. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31001205 Free PMC article. Review.
-
Histological characterization of orphan transporter MCT14 (SLC16A14) shows abundant expression in mouse CNS and kidney.BMC Neurosci. 2016 Jul 1;17(1):43. doi: 10.1186/s12868-016-0274-7. BMC Neurosci. 2016. PMID: 27364523 Free PMC article.
-
Pharmacological treatment and BBB-targeted genetic therapy for MCT8-dependent hypomyelination in zebrafish.Dis Model Mech. 2016 Nov 1;9(11):1339-1348. doi: 10.1242/dmm.027227. Epub 2016 Sep 23. Dis Model Mech. 2016. PMID: 27664134 Free PMC article.
-
Mosaic Expression of Thyroid Hormone Regulatory Genes Defines Cell Type-Specific Dependency in the Developing Chicken Cerebellum.Cerebellum. 2016 Dec;15(6):710-725. doi: 10.1007/s12311-015-0744-y. Cerebellum. 2016. PMID: 26559893
-
Thyroid Hormone Transporters MCT8 and OATP1C1 Are Expressed in Projection Neurons and Interneurons of Basal Ganglia and Motor Thalamus in the Adult Human and Macaque Brains.Int J Mol Sci. 2023 Jun 1;24(11):9643. doi: 10.3390/ijms24119643. Int J Mol Sci. 2023. PMID: 37298594 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

