Membrane binding and bending in Ebola VP40 assembly and egress
- PMID: 24995005
- PMCID: PMC4061899
- DOI: 10.3389/fmicb.2014.00300
Membrane binding and bending in Ebola VP40 assembly and egress
Abstract
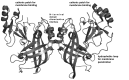
Lipid-enveloped viruses contain a lipid bilayer coat that protects their genome and helps to facilitate entry into the host cell. Filoviruses are lipid-enveloped viruses that have up to 90% clinical fatality and include Marbug (MARV) and Ebola (EBOV). These pleomorphic filamentous viruses enter the host cell through their membrane-embedded glycoprotein and then replicate using just seven genes encoded in their negative-sense RNA genome. EBOV budding occurs from the inner leaflet of the plasma membrane (PM) and is driven by the matrix protein VP40, which is the most abundantly expressed protein of the virus. VP40 expressed in mammalian cells alone can trigger budding of filamentous virus-like particles (VLPs) that are nearly indistinguishable from authentic EBOV. VP40, such as matrix proteins from other viruses, has been shown to bind anionic lipid membranes. However, how VP40 selectively interacts with the inner leaflet of the PM and assembles into a filamentous lipid enveloped particle is mostly unknown. This article describes what is known regarding VP40 membrane interactions and what answers will fill the gaps.
Keywords: Ebola; VP40; filovirus; membrane binding; phosphatidylserine; phosphoinositides; plasma membrane.
Figures

Similar articles
-
The Ebola virus matrix protein VP40 selectively induces vesiculation from phosphatidylserine-enriched membranes.J Biol Chem. 2014 Nov 28;289(48):33590-7. doi: 10.1074/jbc.M114.586396. Epub 2014 Oct 14. J Biol Chem. 2014. PMID: 25315776 Free PMC article.
-
Host Cell Plasma Membrane Phosphatidylserine Regulates the Assembly and Budding of Ebola Virus.J Virol. 2015 Sep;89(18):9440-53. doi: 10.1128/JVI.01087-15. Epub 2015 Jul 1. J Virol. 2015. PMID: 26136573 Free PMC article.
-
Minor electrostatic changes robustly increase VP40 membrane binding, assembly, and budding of Ebola virus matrix protein derived virus-like particles.J Biol Chem. 2024 May;300(5):107213. doi: 10.1016/j.jbc.2024.107213. Epub 2024 Mar 24. J Biol Chem. 2024. PMID: 38522519 Free PMC article.
-
Conformational plasticity of the Ebola virus matrix protein.Protein Sci. 2014 Nov;23(11):1519-27. doi: 10.1002/pro.2541. Epub 2014 Sep 4. Protein Sci. 2014. PMID: 25159197 Free PMC article. Review.
-
Filovirus assembly and budding.Virology. 2006 Jan 5;344(1):64-70. doi: 10.1016/j.virol.2005.09.018. Virology. 2006. PMID: 16364737 Review.
Cited by
-
Mechanisms of phosphatidylserine influence on viral production: A computational model of Ebola virus matrix protein assembly.J Biol Chem. 2022 Jul;298(7):102025. doi: 10.1016/j.jbc.2022.102025. Epub 2022 May 11. J Biol Chem. 2022. PMID: 35568195 Free PMC article.
-
Interdomain salt-bridges in the Ebola virus protein VP40 and their role in domain association and plasma membrane localization.Protein Sci. 2016 Sep;25(9):1648-58. doi: 10.1002/pro.2969. Epub 2016 Jul 4. Protein Sci. 2016. PMID: 27328459 Free PMC article.
-
Negative-sense RNA viruses: An underexplored platform for examining virus-host lipid interactions.Mol Biol Cell. 2021 Oct 1;32(20):pe1. doi: 10.1091/mbc.E19-09-0490. Mol Biol Cell. 2021. PMID: 34570653 Free PMC article. Review.
-
Editorial: Role of lipids in virus assembly.Front Microbiol. 2015 May 5;6:410. doi: 10.3389/fmicb.2015.00410. eCollection 2015. Front Microbiol. 2015. PMID: 25999935 Free PMC article. No abstract available.
-
Nanoscale Mechanical and Morphological Characterization of Ebolavirus-like Particles: Implications for Therapeutic Development.Int J Mol Sci. 2025 May 28;26(11):5185. doi: 10.3390/ijms26115185. Int J Mol Sci. 2025. PMID: 40507994 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

