Dual effects of ouabain on the regulation of proliferation and apoptosis in human umbilical vein endothelial cells: involvement of Na(+)-K(+)-ATPase α-subunits and NF-κB
- PMID: 24995076
- PMCID: PMC4073737
Dual effects of ouabain on the regulation of proliferation and apoptosis in human umbilical vein endothelial cells: involvement of Na(+)-K(+)-ATPase α-subunits and NF-κB
Abstract
Purpose: To elucidate the effect of ouabain on the regulation of proliferation and apoptosis of HUVECs and involvement of different Na(+)-K(+)-ATPase α-subunits and NF-κB.
Methods: HUVECs were isolated by collagenase perfusion, and MTT assays and cell cycle analysis were performed to study proliferation. NF-κB expression and function were examined by immunohistochemical staining and western blotting. Na(+)-K(+)-ATPase activity was determined by measuring released ouabain inhibitable inorganic phosphate (Pi). The expression of different α-subunits was investigated by real RT-PCR, western blotting and cell immunofluorescence.
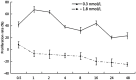
Results: 0.3 nM ouabain treatment for 0.5 h triggered the proliferation of HUVECs, peaking at 1-2 h. At 1.8 nM for 0.5 h, ouabain induced an increase of cell proliferation for a short time, and then triggered a decrease after 1 h. Cell cycle analysis show that 37% of HUVECs were in G2/M phase of the cell cycle following incubation with 1.8 nM ouabain, compared with 18% with 0.3 nM ouabain. NF-κB activity was assessed by western blot analysis of IκB expression, which was significantly reduced with 0.3 nM ouabain treatment; there was no different between 1.8 nM ouabain treatment and untreated cells. Na(+)-K(+)-ATPase activity in HUVECs was markedly reduced after treatment with 0.3 nM and 1.8 nM ouabain. Real RT-PCR and western blotting indicated that Na(+)-K(+)-ATPase α1-subunit mRNA expression levels increased after 0.3 nM ouabain treatment and decreased after 1.8 nM ouabain treatment. However, α2- and α3-subunit mRNA decreased after 0.3 nM ouabain treatment and increased after 1.8 nM ouabain treatment.
Conclusion: Ouabain at different concentrations caused dual effects on proliferation and apoptosis in HUVECs.
Keywords: NF-κB; Ouabain; endothelium; α subunit.
Figures





References
-
- Trevisi L, Pighin I, Luciani S. Vascular endothelium as a target for endogenous ouabain: studies on the effect of ouabain on human endothelial cells. Cell Mol Biol (Noisy-le-grand) 2006;52:64–70. - PubMed
-
- Hamlyn JM, Hamilton BP, Manunta P. Endogenous ouabain, sodium balance and blood pressure: a review and a hypothesis. J Hypertens. 1996;14:151–167. - PubMed
-
- Schoner W, Scheiner-Bobis G. Endogenous cardiac glycosides: hormones using the sodium pump as signal transducer. Semin Nephrol. 2005;25:343–351. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
