Indole alkaloids from marine sources as potential leads against infectious diseases
- PMID: 24995289
- PMCID: PMC4066687
- DOI: 10.1155/2014/375423
Indole alkaloids from marine sources as potential leads against infectious diseases
Abstract
Indole alkaloids comprise a large and complex class of natural products found in a variety of marine sources. Infectious diseases remain a major threat to public health, and in the absence of long-term protective vaccines, the control of these infectious diseases is based on a small number of chemotherapeutic agents. Furthermore, the emerging resistance against these drugs makes it urgently necessary to discover and develop new, safe and, effective anti-infective agents. In this regard, the aim of this review is to highlight indole alkaloids from marine sources which have been shown to demonstrate activity against infectious diseases.
Figures







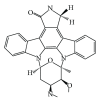


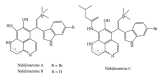



References
-
- Costa-lotufo LV, Wilke DV, Jimenez PC, Epifanio RDA. Marine organisms as a source of new pharmaceuticals: history and perspectives. Química Nova. 2009;32(3):703–716.
-
- Rao JV, Usman PK, Kumar JB. Larvicidal and insecticidal properties of some marine sponges collected in Palk Bay and Gulf of Mannar waters. African Journal of Biotechnology. 2008;7(2):109–113.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

