The cytotoxicity of benzaldehyde nitrogen mustard-2-pyridine carboxylic acid hydrazone being involved in topoisomerase IIα inhibition
- PMID: 24995306
- PMCID: PMC4066686
- DOI: 10.1155/2014/527042
The cytotoxicity of benzaldehyde nitrogen mustard-2-pyridine carboxylic acid hydrazone being involved in topoisomerase IIα inhibition
Abstract
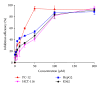
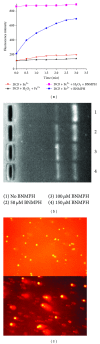
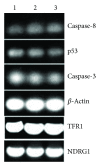
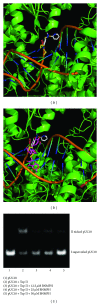
The antitumor property of iron chelators and aromatic nitrogen mustard derivatives has been well documented. Combination of the two pharmacophores in one molecule in drug designation is worth to be explored. We reported previously the syntheses and preliminary cytotoxicity evaluation of benzaldehyde nitrogen mustard pyridine carboxyl acid hydrazones (BNMPH) as extended study, more tumor cell lines (IC50 for HepG2: 26.1 ± 3.5 μM, HCT-116: 57.5 ± 5.3 μM, K562: 48.2 ± 4.0 μM, and PC-12: 19.4 ± 2.2 μM) were used to investigate its cytotoxicity and potential mechanism. In vitro experimental data showed that the BNMPH chelating Fe(2+) caused a large number of ROS formations which led to DNA cleavage, and this was further supported by comet assay, implying that ROS might be involved in the cytotoxicity of BNMPH. The ROS induced changes of apoptosis related genes, but the TFR1 and NDRG1 metastatic genes were not obviously regulated, prompting that BNMPH might not be able to deprive Fe(2+) of ribonucleotide reductase. The BNMPH induced S phase arrest was different from that of iron chelators (G1) and alkylating agents (G2). BNMPH also exhibited its inhibition of human topoisomerase IIα. Those revealed that the cytotoxic mechanism of the BNMPH could stem from both the topoisomerase II inhibition, ROS generation and DNA alkylation.
Figures



Similar articles
-
Redox cycling of a copper complex with benzaldehyde nitrogen mustard-2-pyridine carboxylic acid hydrazone contributes to its enhanced antitumor activity, but no change in the mechanism of action occurs after chelation.Oncol Rep. 2016 Mar;35(3):1636-44. doi: 10.3892/or.2015.4530. Epub 2015 Dec 29. Oncol Rep. 2016. PMID: 26718494
-
Phenolic-containing organic extracts of mulberry (Morus alba L.) leaves inhibit HepG2 hepatoma cells through G2/M phase arrest, induction of apoptosis, and inhibition of topoisomerase IIα activity.J Med Food. 2010 Oct;13(5):1045-56. doi: 10.1089/jmf.2010.1021. J Med Food. 2010. PMID: 20828312
-
The antitumor mechanism of di-2-pyridylketone 2-pyridine carboxylic acid hydrazone and its copper complex in ROS generation and topoisomerase inhibition, and hydrazone involvement in oxygen-catalytic iron mobilization.Int J Oncol. 2015 Nov;47(5):1854-62. doi: 10.3892/ijo.2015.3158. Epub 2015 Sep 11. Int J Oncol. 2015. PMID: 26398524
-
Inhibition of topoisomerase IIα sensitizes FaDu cells to ionizing radiation by diminishing DNA repair.Tumour Biol. 2015 Nov;36(11):8985-92. doi: 10.1007/s13277-015-3587-8. Epub 2015 Jun 17. Tumour Biol. 2015. PMID: 26081617
-
Simultaneous amplification of HER-2 (ERBB2) and topoisomerase IIalpha (TOP2A) genes--molecular basis for combination chemotherapy in cancer.Curr Cancer Drug Targets. 2006 Nov;6(7):579-602. doi: 10.2174/156800906778742497. Curr Cancer Drug Targets. 2006. PMID: 17100565 Review.
References
-
- Chen Y, Hu L. Design of anticancer prodrugs for reductive activation. Medicinal Research Reviews. 2009;29(1):29–64. - PubMed
-
- Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treatment Reviews. 2009;35(1):32–46. - PubMed
-
- Pahl PMB, Horwitz LD. Cell permeable iron chelators as potential cancer chemotherapeutic agents. Cancer Investigation. 2005;23(8):683–691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

