In vitro dissolution and in vivo bioavailability of six brands of ciprofloxacin tablets administered in rabbits and their pharmacokinetic modeling
- PMID: 24995312
- PMCID: PMC4065682
- DOI: 10.1155/2014/590848
In vitro dissolution and in vivo bioavailability of six brands of ciprofloxacin tablets administered in rabbits and their pharmacokinetic modeling
Abstract
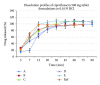
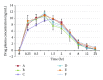
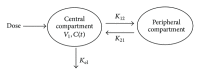
This study was undertaken to assess the in vitro dissolution and in vivo bioavailability of six brands of ciprofloxacin oral tablets available in the UAE market using rabbits. The in vitro dissolution profiles of the six ciprofloxacin products were determined using the USP dissolution paddle method. Pharmacokinetic modeling using compartmental and noncompartmental analysis was done to determine the pharmacokinetic parameters of ciprofloxacin after single-dose oral administration. In vitro release study revealed that the amount of ciprofloxacin released in 20 minutes was not less than 80% of the labeled amount which is in accordance with the pharmacopoeial requirements. All tested products are considered to be very rapid dissolving except for formulae A and D. Ciprofloxacin plasma concentration in rabbits was best fitted to a two-compartment open model. The lowest bioavailability was determined to be for product A (93.24%) while the highest bioavailability was determined to be for product E (108.01%). Postmarketing surveillance is very crucial to ensure product quality and eliminating substandard products to be distributed and, consequently, ensure better patient clinical outcome. The tested ciprofloxacin generic products distributed in the UAE market were proven to be of good quality and could be used interchangeably with the branded ciprofloxacin product.
Figures
Similar articles
-
In vitro quality evaluation of leading brands of ciprofloxacin tablets available in Bangladesh.BMC Res Notes. 2017 May 30;10(1):185. doi: 10.1186/s13104-017-2507-y. BMC Res Notes. 2017. PMID: 28558832 Free PMC article.
-
Ciprofloxacin pharmacokinetics and oral absorption of generic ciprofloxacin tablets in dogs.Am J Vet Res. 2012 Jul;73(7):1085-91. doi: 10.2460/ajvr.73.7.1085. Am J Vet Res. 2012. PMID: 22738062
-
Bioavailability of ciprofloxacin tablets in humans and its correlation with the dissolution rates.Pak J Pharm Sci. 2009 Jul;22(3):329-34. Pak J Pharm Sci. 2009. PMID: 19553184 Clinical Trial.
-
Bioavailability and Bioequivalence Aspects of Oral Modified-Release Drug Products.AAPS J. 2017 Mar;19(2):360-366. doi: 10.1208/s12248-016-0025-9. Epub 2016 Dec 21. AAPS J. 2017. PMID: 28004346 Review.
-
Bootstrap Statistics and Its Application in Disintegration and Dissolution Data Analysis.Mol Pharm. 2023 Aug 7;20(8):3791-3803. doi: 10.1021/acs.molpharmaceut.3c00222. Epub 2023 Jul 17. Mol Pharm. 2023. PMID: 37459158 Review.
Cited by
-
In vitro comparative quality assessment of different brands of norfloxacin tablets available in Jimma, Southwest Ethiopia.Drug Des Devel Ther. 2019 Apr 17;13:1241-1249. doi: 10.2147/DDDT.S189524. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31118565 Free PMC article.
-
Molecular Crystal Forms of Antitubercular Ethionamide with Dicarboxylic Acids: Solid-State Properties and a Combined Structural and Spectroscopic Study.Pharmaceutics. 2020 Aug 28;12(9):818. doi: 10.3390/pharmaceutics12090818. Pharmaceutics. 2020. PMID: 32872201 Free PMC article.
-
In vitro quality evaluation of leading brands of ciprofloxacin tablets available in Bangladesh.BMC Res Notes. 2017 May 30;10(1):185. doi: 10.1186/s13104-017-2507-y. BMC Res Notes. 2017. PMID: 28558832 Free PMC article.
-
Interplay of the Quality of Ciprofloxacin and Antibiotic Resistance in Developing Countries.Front Pharmacol. 2017 Aug 21;8:546. doi: 10.3389/fphar.2017.00546. eCollection 2017. Front Pharmacol. 2017. PMID: 28871228 Free PMC article.
-
Bactericidal Activity of Ceragenin CSA-13 in Cell Culture and in an Animal Model of Peritoneal Infection.Antimicrob Agents Chemother. 2015 Oct;59(10):6274-82. doi: 10.1128/AAC.00653-15. Epub 2015 Jul 27. Antimicrob Agents Chemother. 2015. PMID: 26248361 Free PMC article.
References
-
- Olaniyi A, Babalola C, Oladeinde F, Adegoke A. Towards better quality assurance of drugs. Proceedings of the 4th National Workshop; 2001; pp. 70–73.
-
- Shah H. Generic captures new prescription markets. Perspectives in Pharmacy Economics. 2013;199(4):p. 3.
-
- Remington JP. Remington's Pharmaceutical Sciences. 18th edition. Mack; 1990.
-
- Olaniyi AA. Towards better quality assurance of drugs and food. Proceedings of the National Workshop; 1993; pp. 123–135.
-
- World Health Organization. WHO Medicines Strategy, Countries at the Core 2004–2007. 2004.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

