MiR-15b targets cyclin D1 to regulate proliferation and apoptosis in glioma cells
- PMID: 24995320
- PMCID: PMC4065679
- DOI: 10.1155/2014/687826
MiR-15b targets cyclin D1 to regulate proliferation and apoptosis in glioma cells
Abstract
Aim: To investigate the role and mechanism of miR-15b in the proliferation and apoptosis of glioma.
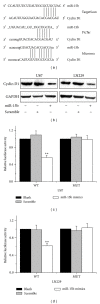
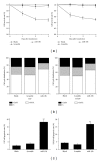
Methods: The miR-15b mimics were transfected into human glioma cells to upregulate the miR-15b expression. Cyclin D1 was determined by both western blotting analysis and luciferase reporter assay. Methylthiazol tetrazolium (MTT) and flow cytometry were employed to detect the cell proliferation, cell cycle, and apoptosis.
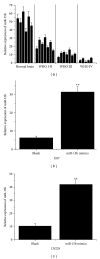
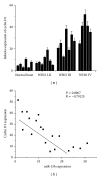
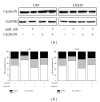
Results: Overexpression of miR-15b inhibits proliferation by arrested cell cycle progression and induces apoptosis, possibly by directly targeting Cyclin D1. Both luciferase assay and bioinformatics search revealed a putative target site of miR-15b binding to the 3'-UTR of Cyclin D1. Moreover, expression of miR-15b in glioma tissues was found to be inversely correlated with Cyclin D1 expression. Enforced Cyclin D1 could abrogate the miR-15b-mediated cell cycle arrest and apoptosis.
Conclusions: Our findings identified that miR-15b may function as a glioma suppressor by targeting the Cyclin D1, which may provide a novel therapeutic strategy for treatment of glioma.
Figures
References
-
- Auffinger B, Thaci B, Ahmed A, Ulasov I, Lesniak MS. MicroRNA targeting as a therapeutic strategy against Glioma. Current Molecular Medicine. 2013;13(4):535–542. - PubMed
-
- Peng B, Li D, Qin M, et al. MicroRNA218 inhibits glioma migration and invasion via inhibiting glioma-associated oncogene homolog 1 expression at N terminus. Tumor Biology. 2014;35(4):3831–3837. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

