Multiple roles of Myd88 in the immune response to the plague F1-V vaccine and in protection against an aerosol challenge of Yersinia pestis CO92 in mice
- PMID: 24995344
- PMCID: PMC4065692
- DOI: 10.1155/2014/341820
Multiple roles of Myd88 in the immune response to the plague F1-V vaccine and in protection against an aerosol challenge of Yersinia pestis CO92 in mice
Abstract
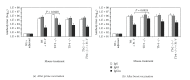
The current candidate vaccine against Yersinia pestis infection consists of two subunit proteins: the capsule protein or F1 protein and the low calcium response V protein or V-antigen. Little is known of the recognition of the vaccine by the host's innate immune system and how it affects the acquired immune response to the vaccine. Thus, we vaccinated Toll-like receptor (Tlr) 2, 4, and 2/4-double deficient, as well as signal adaptor protein Myd88-deficient mice. We found that Tlr4 and Myd88 appeared to be required for an optimal immune response to the F1-V vaccine but not Tlr2 when compared to wild-type mice. However, there was a difference between the requirement for Tlr4 and MyD88 in vaccinated animals. When F1-V vaccinated Tlr4 mutant (lipopolysaccharide tolerant) and Myd88-deficient mice were challenged by aerosol with Y. pestis CO92, all but one Tlr4 mutant mice survived the challenge, but no vaccinated Myd88-deficient mice survived the challenge. Spleens from these latter nonsurviving mice showed that Y. pestis was not cleared from the infected mice. Our results suggest that MyD88 appears to be important for both an optimal immune response to F1-V and in protection against a lethal challenge of Y. pestis CO92 in F1-V vaccinated mice.
Figures


Similar articles
-
Nanolipoprotein particle (NLP) vaccine confers protection against Yersinia pestis aerosol challenge in a BALB/c mouse model.Front Immunol. 2025 Jun 26;16:1603710. doi: 10.3389/fimmu.2025.1603710. eCollection 2025. Front Immunol. 2025. PMID: 40642079 Free PMC article.
-
Evaluation of protective potential of Yersinia pestis outer membrane protein antigens as possible candidates for a new-generation recombinant plague vaccine.Clin Vaccine Immunol. 2013 Feb;20(2):227-38. doi: 10.1128/CVI.00597-12. Epub 2012 Dec 12. Clin Vaccine Immunol. 2013. PMID: 23239803 Free PMC article.
-
Complete Protection against Pneumonic and Bubonic Plague after a Single Oral Vaccination.PLoS Negl Trop Dis. 2015 Oct 16;9(10):e0004162. doi: 10.1371/journal.pntd.0004162. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26473734 Free PMC article.
-
Lipid A mimetics are potent adjuvants for an intranasal pneumonic plague vaccine.Vaccine. 2008 Oct 16;26(44):5554-61. doi: 10.1016/j.vaccine.2008.08.007. Epub 2008 Aug 21. Vaccine. 2008. PMID: 18722493 Free PMC article.
-
Immune defense against pneumonic plague.Immunol Rev. 2008 Oct;225:256-71. doi: 10.1111/j.1600-065X.2008.00674.x. Immunol Rev. 2008. PMID: 18837787 Free PMC article. Review.
Cited by
-
Binding Sites of Anti-Lcr V Monoclonal Antibodies Are More Critical than the Avidities and Affinities for Passive Protection against Yersinia pestis Infection in a Bubonic Plague Model.Antibodies (Basel). 2020 Aug 3;9(3):37. doi: 10.3390/antib9030037. Antibodies (Basel). 2020. PMID: 32756297 Free PMC article.
-
Plague vaccines: new developments in an ongoing search.Appl Microbiol Biotechnol. 2021 Jun;105(12):4931-4941. doi: 10.1007/s00253-021-11389-6. Epub 2021 Jun 18. Appl Microbiol Biotechnol. 2021. PMID: 34142207 Free PMC article. Review.
References
-
- Haffkine WM. Remarks on the plague prophylactic fluid. British Medical Journal. 1897;1:1461–1462.
-
- Williamson ED, Eley SM, Griffin KF, et al. A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunology and Medical Microbiology. 1995;12(3-4):223–230. - PubMed
-
- Heath DG, Anderson GW, Welkos SL, Andrews GP, Friedlander AM, Mauro JM. A recombinant capsular F1-V antigen fusion protein vaccine protects against experimental bubonic and pneumonic plague. In: Brown F, Burton D, Doherty P, Mekalanos J, Norrby E, editors. Vaccine 97. New York, NY, USA: Cold Spring Harbor Laboratory Press; 1997. pp. 197–200.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

