Molecular mediators governing iron-copper interactions
- PMID: 24995690
- PMCID: PMC4316823
- DOI: 10.1146/annurev-nutr-071812-161215
Molecular mediators governing iron-copper interactions
Abstract
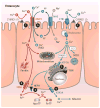
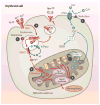
Given their similar physiochemical properties, it is a logical postulate that iron and copper metabolism are intertwined. Indeed, iron-copper interactions were first documented over a century ago, but the homeostatic effects of one on the other has not been elucidated at a molecular level to date. Recent experimental work has, however, begun to provide mechanistic insight into how copper influences iron metabolism. During iron deficiency, elevated copper levels are observed in the intestinal mucosa, liver, and blood. Copper accumulation and/or redistribution within enterocytes may influence iron transport, and high hepatic copper may enhance biosynthesis of a circulating ferroxidase, which potentiates iron release from stores. Moreover, emerging evidence has documented direct effects of copper on the expression and activity of the iron-regulatory hormone hepcidin. This review summarizes current experimental work in this field, with a focus on molecular aspects of iron-copper interplay and how these interactions relate to various disease states.
Keywords: ceruloplasmin, hephaestin; copper-transporting ATPase1; divalent metal-ion transporter 1; ferroportin 1; intestine; liver.
Figures


References
-
- Aigner E, Theurl I, Haufe H, Seifert M, Hohla F, et al. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology. 2008;135:680–88. - PubMed
-
- Arredondo M, Mendiburo MJ, Flores S, Singleton ST, Garrick MD. Mouse divalent metal transporter 1 is a copper transporter in HEK293 cells. Biometals. 2014;27:115–23. - PubMed
-
- Arredondo M, Muñoz P, Mura CV, Núñez MT. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol. 2003;284:C1525–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

