A distinct subfraction of Aβ is responsible for the high-affinity Pittsburgh compound B-binding site in Alzheimer's disease brain
- PMID: 24995708
- PMCID: PMC4205261
- DOI: 10.1111/jnc.12815
A distinct subfraction of Aβ is responsible for the high-affinity Pittsburgh compound B-binding site in Alzheimer's disease brain
Abstract
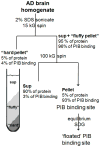
The positron emission tomography (PET) ligand (11) C-labeled Pittsburgh compound B (PIB) is used to image β-amyloid (Aβ) deposits in the brains of living subjects with the intent of detecting early stages of Alzheimer's disease (AD). However, deposits of human-sequence Aβ in amyloid precursor protein transgenic mice and non-human primates bind very little PIB. The high stoichiometry of PIB:Aβ binding in human AD suggests that the PIB-binding site may represent a particularly pathogenic entity and/or report local pathologic conditions. In this study, (3) H-PIB was employed to track purification of the PIB-binding site in > 90% yield from frontal cortical tissue of autopsy-diagnosed AD subjects. The purified PIB-binding site comprises a distinct, highly insoluble subfraction of the Aβ in AD brain with low buoyant density because of the sodium dodecyl sulfate-resistant association with a limited subset of brain proteins and lipids with physical properties similar to lipid rafts and to a ganglioside:Aβ complex in AD and Down syndrome brain. Both the protein and lipid components are required for PIB binding. Elucidation of human-specific biological components and pathways will be important in guiding improvement of the animal models for AD and in identifying new potential therapeutic avenues. A lipid-associated subpopulation of Aβ accounts for the high-affinity binding of Pittsburgh compound B (PIB) in Alzheimer's disease brain. Mass spectrometry of the isolated PIB-binding site from frontal cortex identified Aβ peptides and a set of plaque-associated proteins in AD but not age-matched normal brain. The PIB-binding site may represent a particularly pathogenic entity and/or report local pathologic conditions.
Keywords: ELISA; amyloid; lipids; plaque; radioreceptor assay; tau.
© 2014 International Society for Neurochemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR020171/RR/NCRR NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- P51OD11132/OD/NIH HHS/United States
- R21 NS080576/NS/NINDS NIH HHS/United States
- P51 RR000165/RR/NCRR NIH HHS/United States
- R21 AG040589/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- R21AG040589/AG/NIA NIH HHS/United States
- P51RR165/RR/NCRR NIH HHS/United States
- P20RR020171/RR/NCRR NIH HHS/United States
- P30AG028383/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- P50AG025688/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

