Preserving human cells for regenerative, reproductive, and transfusion medicine
- PMID: 24995723
- PMCID: PMC4145864
- DOI: 10.1002/biot.201300074
Preserving human cells for regenerative, reproductive, and transfusion medicine
Abstract
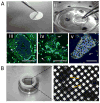
Cell cryopreservation maintains cellular life at sub-zero temperatures by slowing down biochemical processes. Various cell types are routinely cryopreserved in modern reproductive, regenerative, and transfusion medicine. Current cell cryopreservation methods involve freezing (slow/rapid) or vitrifying cells in the presence of a cryoprotective agent (CPA). Although these methods are clinically utilized, cryo-injury due to ice crystals, osmotic shock, and CPA toxicity cause loss of cell viability and function. Recent approaches using minimum volume vitrification provide alternatives to the conventional cryopreservation methods. Minimum volume vitrification provides ultra-high cooling and rewarming rates that enable preserving cells without ice crystal formation. Herein, we review recent advances in cell cryopreservation technology and provide examples of techniques that are utilized in oocyte, stem cell, and red blood cell cryopreservation.
Keywords: Cryopreservation; Regenerative medicine; Reproductive medicine; Transfusion medicine; Vitrification.
Copyright © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Dr. Utkan Demirci is a founder of, and has an equity interest in: (i) DxNow Inc., a company that is developing microfluidic and imaging technologies for point-of-care diagnostic solutions, and (ii) Koek Biotech, a company that is developing microfluidic IVF technologies for clinical solutions. Dr. Utkan Demirci’s interests were viewed and managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

