Aicardi-Goutières syndrome is caused by IFIH1 mutations
- PMID: 24995871
- PMCID: PMC4085581
- DOI: 10.1016/j.ajhg.2014.06.007
Aicardi-Goutières syndrome is caused by IFIH1 mutations
Abstract
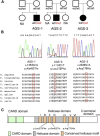
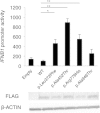
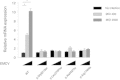
Aicardi-Goutières syndrome (AGS) is a rare, genetically determined early-onset progressive encephalopathy. To date, mutations in six genes have been identified as etiologic for AGS. Our Japanese nationwide AGS survey identified six AGS-affected individuals without a molecular diagnosis; we performed whole-exome sequencing on three of these individuals. After removal of the common polymorphisms found in SNP databases, we were able to identify IFIH1 heterozygous missense mutations in all three. In vitro functional analysis revealed that IFIH1 mutations increased type I interferon production, and the transcription of interferon-stimulated genes were elevated. IFIH1 encodes MDA5, and mutant MDA5 lacked ligand-specific responsiveness, similarly to the dominant Ifih1 mutation responsible for the SLE mouse model that results in type I interferon overproduction. This study suggests that the IFIH1 mutations are responsible for the AGS phenotype due to an excessive production of type I interferon.
Copyright © 2014 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Autosomal dominant IFIH1 gain-of-function mutations cause Aicardi-Goutières syndrome.Clin Genet. 2014 Nov;86(5):473-4. doi: 10.1111/cge.12471. Epub 2014 Sep 8. Clin Genet. 2014. PMID: 25080300
References
-
- Chahwan C., Chahwan R. Aicardi-Goutieres syndrome: from patients to genes and beyond. Clin. Genet. 2012;81:413–420. - PubMed
-
- Ramantani G., Kohlhase J., Hertzberg C., Innes A.M., Engel K., Hunger S., Borozdin W., Mah J.K., Ungerath K., Walkenhorst H. Expanding the phenotypic spectrum of lupus erythematosus in Aicardi-Goutières syndrome. Arthritis Rheum. 2010;62:1469–1477. - PubMed
-
- Orcesi S., La Piana R., Fazzi E. Aicardi-Goutieres syndrome. Br. Med. Bull. 2009;89:183–201. - PubMed
-
- Blau N., Bonafé L., Krägeloh-Mann I., Thöny B., Kierat L., Häusler M., Ramaekers V. Cerebrospinal fluid pterins and folates in Aicardi-Goutières syndrome: a new phenotype. Neurology. 2003;61:642–647. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

