Cardiac mitochondrial proteome dynamics with heavy water reveals stable rate of mitochondrial protein synthesis in heart failure despite decline in mitochondrial oxidative capacity
- PMID: 24995939
- PMCID: PMC5363075
- DOI: 10.1016/j.yjmcc.2014.06.014
Cardiac mitochondrial proteome dynamics with heavy water reveals stable rate of mitochondrial protein synthesis in heart failure despite decline in mitochondrial oxidative capacity
Abstract
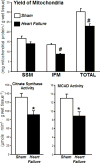
We recently developed a method to measure mitochondrial proteome dynamics with heavy water ((2)H2O)-based metabolic labeling and high resolution mass spectrometry. We reported the half-lives and synthesis rates of several proteins in the two cardiac mitochondrial subpopulations, subsarcolemmal and interfibrillar (SSM and IFM), in Sprague Dawley rats. In the present study, we tested the hypothesis that the mitochondrial protein synthesis rate is reduced in heart failure, with possible differential changes in SSM versus IFM. Six to seven week old male Sprague Dawley rats underwent transverse aortic constriction (TAC) and developed moderate heart failure after 22weeks. Heart failure and sham rats of the same age received heavy water (5% in drinking water) for up to 80days. Cardiac SSM and IFM were isolated from both groups and the proteins were separated by 1D gel electrophoresis. Heart failure reduced protein content and increased the turnover rate of several proteins involved in fatty acid oxidation, electron transport chain and ATP synthesis, while it decreased the turnover of other proteins, including pyruvate dehydrogenase subunit in IFM, but not in SSM. Because of these bidirectional changes, the average overall half-life of proteins was not altered by heart failure in both SSM and IFM. The kinetic measurements of individual mitochondrial proteins presented in this study may contribute to a better understanding of the mechanisms responsible for mitochondrial alterations in the failing heart.
Keywords: Cardiac failure; Deuterium; Mitochondria; Proteome dynamics.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest, financial or otherwise.
Figures





Similar articles
-
Assessment of cardiac proteome dynamics with heavy water: slower protein synthesis rates in interfibrillar than subsarcolemmal mitochondria.Am J Physiol Heart Circ Physiol. 2013 May;304(9):H1201-14. doi: 10.1152/ajpheart.00933.2012. Epub 2013 Mar 1. Am J Physiol Heart Circ Physiol. 2013. PMID: 23457012 Free PMC article.
-
Pressure overload differentially affects respiratory capacity in interfibrillar and subsarcolemmal mitochondria.Am J Physiol Heart Circ Physiol. 2013 Feb 15;304(4):H529-37. doi: 10.1152/ajpheart.00699.2012. Epub 2012 Dec 15. Am J Physiol Heart Circ Physiol. 2013. PMID: 23241325
-
Cardiac subsarcolemmal and interfibrillar mitochondria display distinct responsiveness to protection by diazoxide.PLoS One. 2012;7(9):e44667. doi: 10.1371/journal.pone.0044667. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22973464 Free PMC article.
-
Mitochondrial Bioenergetics and Dysfunction in Failing Heart.Adv Exp Med Biol. 2017;982:65-80. doi: 10.1007/978-3-319-55330-6_4. Adv Exp Med Biol. 2017. PMID: 28551782 Review.
-
Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies.Am J Physiol Heart Circ Physiol. 2014 Jul 1;307(1):H1-14. doi: 10.1152/ajpheart.00747.2013. Am J Physiol Heart Circ Physiol. 2014. PMID: 24778166 Free PMC article. Review.
Cited by
-
Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement From the American Heart Association.Circ Res. 2016 Jun 10;118(12):1960-91. doi: 10.1161/RES.0000000000000104. Epub 2016 Apr 28. Circ Res. 2016. PMID: 27126807 Free PMC article. Review.
-
Blueprint of the distinct metabolite profiles of healthy pig heart chambers.J Mol Cell Cardiol Plus. 2025 Jun 10;13:100462. doi: 10.1016/j.jmccpl.2025.100462. eCollection 2025 Sep. J Mol Cell Cardiol Plus. 2025. PMID: 40606519 Free PMC article.
-
Sex differences in the regulation of spatially distinct cardiac mitochondrial subpopulations.Mol Cell Biochem. 2016 Aug;419(1-2):41-51. doi: 10.1007/s11010-016-2748-4. Epub 2016 Jul 2. Mol Cell Biochem. 2016. PMID: 27370644
-
Cardiovascular proteomics in the era of big data: experimental and computational advances.Clin Proteomics. 2016 Dec 5;13:23. doi: 10.1186/s12014-016-9124-y. eCollection 2016. Clin Proteomics. 2016. PMID: 27980500 Free PMC article. Review.
-
Gaussian Process Modeling of Protein Turnover.J Proteome Res. 2016 Jul 1;15(7):2115-22. doi: 10.1021/acs.jproteome.5b00990. Epub 2016 Jun 9. J Proteome Res. 2016. PMID: 27229456 Free PMC article.
References
-
- Bugger H, Schwarzer M, Chen D, Schrepper A, Amorim PA, Schoepe M, et al. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovascular research. 2010;85:376–84. - PubMed
-
- Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. Journal of molecular and cellular cardiology. 1992;24:1333–47. - PubMed
-
- Schwarzer M, Schrepper A, Amorim PA, Osterholt M, Doenst T. Pressure overload differentially affects respiratory capacity in interfibrillar and subsarcolemmal mitochondria. American journal of physiology Heart and circulatory physiology. 2013;304:H529–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

