Immune modulation of stem cells and regeneration
- PMID: 24996166
- PMCID: PMC4131296
- DOI: 10.1016/j.stem.2014.06.009
Immune modulation of stem cells and regeneration
Abstract
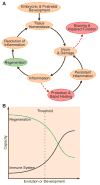
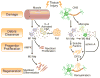
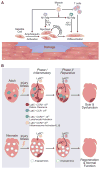
The immune system, best known as the first line of defense against invading pathogens, is integral to tissue development, homeostasis, and wound repair. In recent years, there has been a growing appreciation that cellular and humoral components of the immune system also contribute to regeneration of damaged tissues, including limbs, skeletal muscle, heart, and the nervous system. Here, we discuss key findings that implicate inflammatory cells and their secreted factors in tissue replacement after injury via stem cells and other reparative mechanisms. We highlight clinical conditions that are amenable to immune-mediated regeneration and suggest immune targeting strategies for tissue regeneration.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
References
-
- Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, Polak M. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol. 2004;76:359–367. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

