Quality of life in purely ocular myasthenia in Japan
- PMID: 24996227
- PMCID: PMC4088302
- DOI: 10.1186/1471-2377-14-142
Quality of life in purely ocular myasthenia in Japan
Abstract
Background: Since there has been no conclusive evidence regarding the treatment of ocular myasthenia, treatment guidelines were recently issued by the European Federation of Neurological Societies/European Neurological Society (EFNS/ENS). However, the therapeutic outcomes concerning the quality-of-life (QOL) of patients with ocular myasthenia are not yet fully understood.
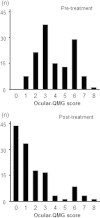
Methods: We investigated the therapeutic outcomes of patients with purely ocular myasthenia in a multicenter cross-sectional survey in Japan. To evaluate the severity of ocular symptoms, we used the ocular-quantitative MG (QMG) score advocated by Myasthenia Gravis Foundation of America. We used the Japanese translated version of the MG-QOL15, a self-appraised scoring system.
Results: Of 607 myasthenia gravis (MG) patients with an observation-duration of illness ≥ 2 years, the cases of 123 patients (20%) were limited to ocular muscles (purely ocular myasthenia). During the entire clinical course, 81 patients experienced both ptosis and diplopia, 36 had ptosis alone, and six had diplopia alone. Acetyl-cholinesterase inhibitors and prednisolone were used in 98 and 52 patients, respectively. Treatment improved ocular symptoms, with the mean reduction in ocular-QMG score of 2.3 ± 1.8 points. However, 47 patients (38%) failed to gain minimal manifestation or a better status. Patients with unfavorable outcomes also self-reported severe QOL impairment. Multivariate analyses showed that the pretreatment ocular-QMG score was associated with unfavorable outcomes, but not associated with the patient's QOL.
Conclusion: A treatment strategy designed in accord with a patient's ocular presentation must be considered in order to improve ocular symptoms and the patient's QOL.
Figures

References
-
- Evoli A, Batocchi AP, Minisci C, Di Schino C, Tonali P. Therapeutic options in ocular myasthenia gravis. Neuromuscul Disord. 2001;11:208–216. - PubMed
-
- Kaminski HJ, Li Z, Richmonds C, Lin F, Medof ME. Complement regulators in extraocular muscle and experimental autoimmune myasthenia gravis. Exp Neurol. 2004;189:333–342. - PubMed
-
- Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37:141–149. - PubMed
-
- Bever CT Jr, Aquino AV, Penn AS, Lovelace RE, Rowland LP. Prognosis of ocular myasthenia. Ann Neurol. 1983;14:516–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

