Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial: design, rationale and implementation
- PMID: 24996573
- PMCID: PMC4137482
- DOI: 10.1016/j.injury.2014.06.001
Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial: design, rationale and implementation
Abstract
Background: Forty percent of in-hospital deaths among injured patients involve massive truncal haemorrhage. These deaths may be prevented with rapid haemorrhage control and improved resuscitation techniques. The Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial was designed to determine if there is a difference in mortality between subjects who received different ratios of FDA approved blood products. This report describes the design and implementation of PROPPR.
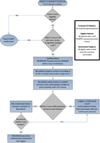
Study design: PROPPR was designed as a randomized, two-group, Phase III trial conducted in subjects with the highest level of trauma activation and predicted to have a massive transfusion. Subjects at 12 North American level 1 trauma centres were randomized into one of two standard transfusion ratio interventions: 1:1:1 or 1:1:2, (plasma, platelets, and red blood cells). Clinical data and serial blood samples were collected under Exception from Informed Consent (EFIC) regulations. Co-primary mortality endpoints of 24h and 30 days were evaluated.
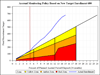
Results: Between August 2012 and December 2013, 680 patients were randomized. The overall median time from admission to randomization was 26min. PROPPR enrolled at higher than expected rates with fewer than expected protocol deviations.
Conclusion: PROPPR is the largest randomized study to enrol severely bleeding patients. This study showed that rapidly enrolling and successfully providing randomized blood products to severely injured patients in an EFIC study is feasible. PROPPR was able to achieve these goals by utilizing a collaborative structure and developing successful procedures and design elements that can be part of future trauma studies.
Trial registration: ClinicalTrials.gov NCT01545232.
Keywords: Clinical trial; Plasma; Platelets; Resuscitation; Transfusion; Trauma.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
No conflicts of interest have been declared by any author in regards to this manuscript.
Figures


References
-
- Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. - PubMed
-
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. - PubMed
-
- Perkins JG, Cap AP, Spinella PC, Blackbourne LH, Grathwohl KW, Repine TB, et al. An evaluation of the impact of apheresis platelets used in the setting of massively transfused trauma patients. J Trauma. 2009;66:S77–S84. discussion S-5. - PubMed
-
- Demetriades D, Murray J, Charalambides K, Alo K, Velmahos G, Rhee P, et al. Trauma fatalities: time and location of hospital deaths. J Am Coll Surg. 2004;198:20–26. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

