TIR1-like auxin-receptors are involved in the regulation of plum fruit development
- PMID: 24996652
- PMCID: PMC4157706
- DOI: 10.1093/jxb/eru279
TIR1-like auxin-receptors are involved in the regulation of plum fruit development
Abstract
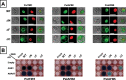
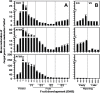
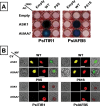
Ethylene has long been considered the key regulator of ripening in climacteric fruit. Recent evidence showed that auxin also plays an important role during fruit ripening, but the nature of the interaction between the two hormones has remained unclear. To understand the differences in ethylene- and auxin-related behaviours that might reveal how the two hormones interact, we compared two plum (Prunus salicina L.) cultivars with widely varying fruit development and ripening ontogeny. The early-ripening cultivar, Early Golden (EG), exhibited high endogenous auxin levels and auxin hypersensitivity during fruit development, while the late-ripening cultivar, V98041 (V9), displayed reduced auxin content and sensitivity. We show that exogenous auxin is capable of dramatically accelerating fruit development and ripening in plum, indicating that this hormone is actively involved in the ripening process. Further, we demonstrate that the variations in auxin sensitivity between plum cultivars could be partially due to PslAFB5, which encodes a TIR1-like auxin receptor. Two different PslAFB5 alleles were identified, one (Pslafb5) inactive due to substitution of the conserved F-box amino acid residue Pro61 to Ser. The early-ripening cultivar, EG, exhibited homozygosity for the inactive allele; however, the late cultivar, V9, displayed a PslAFB5/afb5 heterozygous genotype. Our results highlight the impact of auxin in stimulating fruit development, especially the ripening process and the potential for differential auxin sensitivity to alter important fruit developmental processes.
Keywords: Auxin-receptors; Prunus salicina; auxin/ethylene crosstalk.; plum fruit development; protein–protein interaction; subcellular localization.
© The Author 2014. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




Similar articles
-
Expression of auxin-binding protein1 during plum fruit ontogeny supports the potential role of auxin in initiating and enhancing climacteric ripening.Plant Cell Rep. 2012 Oct;31(10):1911-21. doi: 10.1007/s00299-012-1304-2. Epub 2012 Jun 28. Plant Cell Rep. 2012. PMID: 22739723
-
Regulation of two germin-like protein genes during plum fruit development.J Exp Bot. 2010 Jun;61(6):1761-70. doi: 10.1093/jxb/erq043. Epub 2010 Mar 4. J Exp Bot. 2010. PMID: 20202999 Free PMC article.
-
Hormone balance in a climacteric plum fruit and its non-climacteric bud mutant during ripening.Plant Sci. 2019 Mar;280:51-65. doi: 10.1016/j.plantsci.2018.11.001. Epub 2018 Nov 14. Plant Sci. 2019. PMID: 30824029
-
Differential regulation of four members of the ACC synthase gene family in plum.J Exp Bot. 2008;59(8):2009-27. doi: 10.1093/jxb/ern056. J Exp Bot. 2008. PMID: 18535295 Free PMC article.
-
Molecular Bases of Fruit Quality in Prunus Species: An Integrated Genomic, Transcriptomic, and Metabolic Review with a Breeding Perspective.Int J Mol Sci. 2020 Dec 30;22(1):333. doi: 10.3390/ijms22010333. Int J Mol Sci. 2020. PMID: 33396946 Free PMC article. Review.
Cited by
-
Bioinformatics Study of Aux/IAA Family Genes and Their Expression in Response to Different Hormones Treatments during Japanese Apricot Fruit Development and Ripening.Plants (Basel). 2022 Jul 22;11(15):1898. doi: 10.3390/plants11151898. Plants (Basel). 2022. PMID: 35893602 Free PMC article.
-
Molecular mapping of genomic regions and identification of possible candidate genes associated with gynoecious sex expression in bitter gourd.Front Plant Sci. 2023 Mar 2;14:1071648. doi: 10.3389/fpls.2023.1071648. eCollection 2023. Front Plant Sci. 2023. PMID: 36938036 Free PMC article.
-
Recent Advances in Phytohormone Regulation of Apple-Fruit Ripening.Plants (Basel). 2021 Sep 30;10(10):2061. doi: 10.3390/plants10102061. Plants (Basel). 2021. PMID: 34685870 Free PMC article. Review.
-
Overexpression of plum auxin receptor PslTIR1 in tomato alters plant growth, fruit development and fruit shelf-life characteristics.BMC Plant Biol. 2016 Feb 29;16:56. doi: 10.1186/s12870-016-0746-z. BMC Plant Biol. 2016. PMID: 26927309 Free PMC article.
-
The molecular mechanism on suppression of climacteric fruit ripening with postharvest wax coating treatment via transcriptome.Front Plant Sci. 2022 Aug 15;13:978013. doi: 10.3389/fpls.2022.978013. eCollection 2022. Front Plant Sci. 2022. PMID: 36046594 Free PMC article.
References
-
- Agustí M, Almela V, Andreu I, Juan M, Zacarias L. 1999. Synthetic auxin 3,5,6-TPA promotes fruit development and climacteric in Prunus persica L. Batsch. Journal of Horticultural Science and Biotechnology 74, 556–560.
-
- Cheng Y, Zhao Y. 2007. A role for auxin in flower development. Journal of Integrative Plant Biology 49, 99–104.
-
- Coen ES. 1992. Flower development. Current Opinion in Cell Biology 4, 929–933. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

