Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6
- PMID: 24996823
- PMCID: PMC4181451
- DOI: 10.1093/glycob/cwu064
Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6
Abstract
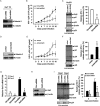
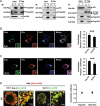
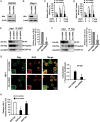
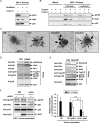
Galectin-3 has been reported to regulate the functions of a number of immune cell types. We previously reported that galectin-3 is translocated to immunological synapses in T cells upon T-cell receptor engagement, where it associates with ALG-2-interacting protein X (Alix). Alix is known to coordinate with the endosomal sorting complex required for transport (ESCRT) to promote human immunodeficiency virus (HIV)-1 virion release. We hypothesized that galectin-3 plays a role in HIV-1 viral budding. Cotransfection of cells of the Jurkat T line with galectin-3 and HIV-1 plasmids resulted in increased HIV-1 budding, and suppression of galectin-3 expression by RNAi in Hut78 and primary CD4+ T cells led to reduced HIV-1 budding. We used immunofluorescence microscopy to observe the partial colocalization of galectin-3, Alix and Gag in HIV-1-infected cells. Results from co-immunoprecipitation experiments indicate that galectin-3 expression promotes Alix-Gag p6 association, whereas the results of Alix knockdown suggest that galectin-3 promotes HIV-1 budding through Alix. HIV-1 particles released from galectin-3-expressing cells acquire the galectin-3 protein in an Alix-dependent manner, with proteins primarily residing inside the virions. We also found that the galectin-3 N-terminal domain interacts with the proline-rich region of Alix. Collectively, these results suggest that endogenous galectin-3 facilitates HIV-1 budding by promoting the Alix-Gag p6 association.
Keywords: Alix; HIV-1; galectin-3; viral budding.
© The Author 2014. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
HIV-1 Subtype C with PYxE Insertion Has Enhanced Binding of Gag-p6 to Host Cell Protein ALIX and Increased Replication Fitness.J Virol. 2019 Apr 17;93(9):e00077-19. doi: 10.1128/JVI.00077-19. Print 2019 May 1. J Virol. 2019. PMID: 30760577 Free PMC article.
-
The ESCRT-associated protein Alix recruits the ubiquitin ligase Nedd4-1 to facilitate HIV-1 release through the LYPXnL L domain motif.J Virol. 2010 Aug;84(16):8181-92. doi: 10.1128/JVI.00634-10. Epub 2010 Jun 2. J Virol. 2010. PMID: 20519395 Free PMC article.
-
Alix-Mediated Rescue of Feline Immunodeficiency Virus Budding Differs from That Observed with Human Immunodeficiency Virus.J Virol. 2020 May 18;94(11):e02019-19. doi: 10.1128/JVI.02019-19. Print 2020 May 18. J Virol. 2020. PMID: 32213612 Free PMC article.
-
The HIV-1 gag p6: a promising target for therapeutic intervention.Retrovirology. 2024 Jan 23;21(1):1. doi: 10.1186/s12977-024-00633-2. Retrovirology. 2024. PMID: 38263239 Free PMC article. Review.
-
ALIX and the multivesicular endosome: ALIX in Wonderland.Trends Cell Biol. 2014 Jan;24(1):19-25. doi: 10.1016/j.tcb.2013.10.009. Epub 2013 Nov 26. Trends Cell Biol. 2014. PMID: 24287454 Review.
Cited by
-
Human lectins and their roles in viral infections.Molecules. 2015 Jan 29;20(2):2229-71. doi: 10.3390/molecules20022229. Molecules. 2015. PMID: 25642836 Free PMC article. Review.
-
Synthesis, Assembly and Processing of Viral Proteins.Viruses. 2022 Jul 27;14(8):1650. doi: 10.3390/v14081650. Viruses. 2022. PMID: 36016272 Free PMC article.
-
Targeting Myeloid Differentiation Primary Response Protein 88 (MyD88) and Galectin-3 to Develop Broad-Spectrum Host-Mediated Therapeutics against SARS-CoV-2.Int J Mol Sci. 2024 Aug 1;25(15):8421. doi: 10.3390/ijms25158421. Int J Mol Sci. 2024. PMID: 39125989 Free PMC article. Review.
-
The Antiviral Role of Galectins toward Influenza A Virus Infection-An Alternative Strategy for Influenza Therapy.Pharmaceuticals (Basel). 2021 May 20;14(5):490. doi: 10.3390/ph14050490. Pharmaceuticals (Basel). 2021. PMID: 34065500 Free PMC article. Review.
-
Upregulation of galectin-3 in influenza A virus infection promotes viral RNA synthesis through its association with viral PA protein.J Biomed Sci. 2023 Feb 23;30(1):14. doi: 10.1186/s12929-023-00901-x. J Biomed Sci. 2023. PMID: 36823664 Free PMC article.
References
-
- Caballe A, Martin-Serrano J. ESCRT machinery and cytokinesis: The road to daughter cell separation. Traffic. 2011;12:1318–1326. - PubMed
-
- Chen C, Vincent O, Jin J, Weisz OA, Montelaro RC. Functions of early (AP-2) and late (AIP1/ALIX) endocytic proteins in equine infectious anemia virus budding. J Biol Chem. 2005;280:40474–40480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

