Epigenetic variation in the mu-opioid receptor gene in infants with neonatal abstinence syndrome
- PMID: 24996986
- PMCID: PMC4145036
- DOI: 10.1016/j.jpeds.2014.05.040
Epigenetic variation in the mu-opioid receptor gene in infants with neonatal abstinence syndrome
Abstract
Objective: Neonatal abstinence syndrome (NAS) from in utero opioid exposure is highly variable with genetic factors appearing to play an important role. Epigenetic changes in cytosine:guanine (CpG) dinucleotide methylation can occur after drug exposure and may help to explain NAS variability. We correlated DNA methylation levels in the mu-opioid receptor (OPRM1) promoter in opioid-exposed infants with NAS outcomes.
Study design: DNA samples from cord blood or saliva were analyzed for 86 infants who were being treated for NAS according to institutional protocol. Methylation levels at 16 OPRM1 CpG sites were determined and correlated with NAS outcome measures, including need for treatment, treatment with ≥ 2 medications, and length of hospital stay. We adjusted for covariates and multiple genetic testing.
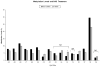
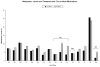
Results: Sixty-five percent of infants required treatment for NAS, and 24% required ≥ 2 medications. Hypermethylation of the OPRM1 promoter was measured at the -10 CpG in treated vs nontreated infants (adjusted difference δ = 3.2% [95% CI, 0.3-6.0%], P = .03; nonsignificant after multiple testing correction). There was hypermethylation at the -14 (δ = 4.9% [95% CI, 1.8%-8.1%], P = .003), -10 (δ = 5.0% [95% CI, 2.3-7.7%], P = .0005), and +84 (δ = 3.5% [95% CI, 0.6-6.4], P = .02) CpG sites in infants requiring ≥ 2 medications, which remained significant for -14 and -10 after multiple testing correction.
Conclusions: Increased methylation within the OPRM1 promoter is associated with worse NAS outcomes, consistent with gene silencing.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
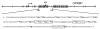
References
-
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000 – 2009. JAMA. 2012;307:1934–40. - PubMed
-
- U.S. Department of Health and Human Services, Substance Abuse and Mental Health Administration. Results from the 2011 National Survey on Drug Use and Health: National Findings. 2011.
-
- Hudak ML, Tan RC. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

