Antiviral drugs specific for coronaviruses in preclinical development
- PMID: 24997250
- PMCID: PMC4195804
- DOI: 10.1016/j.coviro.2014.06.002
Antiviral drugs specific for coronaviruses in preclinical development
Abstract
Coronaviruses are positive stranded RNA viruses that cause respiratory, enteric and central nervous system diseases in many species, including humans. Until recently, the relatively low burden of disease in humans caused by few of these viruses impeded the development of coronavirus specific therapeutics. However, the emergence of severe acute respiratory syndrome coronavirus (SARS-CoV), and more recently, Middle East respiratory syndrome coronavirus (MERS-CoV), has impelled the development of such drugs. This review focuses on some newly identified SARS-CoV inhibitors, with known mechanisms of action and their potential to inhibit the novel MERS-CoV. The clinical development of optimized versions of such compounds could be beneficial for the treatment and control of SARS-CoV, the current MERS-CoV and other future SARS-like epidemics.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
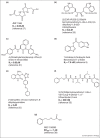
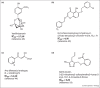
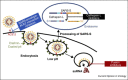
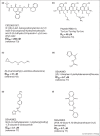
References
-
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. - PubMed
-
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. - PubMed
-
This is the first report for the complete sequence of SARS-CoV complete genome with phylogenetic analyses and sequence comparisons indicating its novelty.
-
- Thiel V., Ivanov K.A., Putics A., Hertzig T., Schelle B., Bayer S., Weissbrich B., Snijder E.J., Rabenau H., Doerr H.W. Mechanisms and enzymes involved in SARS coronavirus genome expression. J Gen Virol. 2003;84:2305–2315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

