Cortical reorganization after spinal cord injury: always for good?
- PMID: 24997269
- PMCID: PMC4556279
- DOI: 10.1016/j.neuroscience.2014.06.056
Cortical reorganization after spinal cord injury: always for good?
Abstract
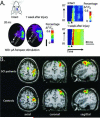
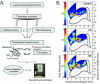
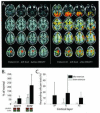
Plasticity constitutes the basis of behavioral changes as a result of experience. It refers to neural network shaping and re-shaping at the global level and to synaptic contacts remodeling at the local level, either during learning or memory encoding, or as a result of acute or chronic pathological conditions. 'Plastic' brain reorganization after central nervous system lesions has a pivotal role in the recovery and rehabilitation of sensory and motor dysfunction, but can also be "maladaptive". Moreover, it is clear that brain reorganization is not a "static" phenomenon but rather a very dynamic process. Spinal cord injury immediately initiates a change in brain state and starts cortical reorganization. In the long term, the impact of injury - with or without accompanying therapy - on the brain is a complex balance between supraspinal reorganization and spinal recovery. The degree of cortical reorganization after spinal cord injury is highly variable, and can range from no reorganization (i.e. "silencing") to massive cortical remapping. This variability critically depends on the species, the age of the animal when the injury occurs, the time after the injury has occurred, and the behavioral activity and possible therapy regimes after the injury. We will briefly discuss these dependencies, trying to highlight their translational value. Overall, it is not only necessary to better understand how the brain can reorganize after injury with or without therapy, it is also necessary to clarify when and why brain reorganization can be either "good" or "bad" in terms of its clinical consequences. This information is critical in order to develop and optimize cost-effective therapies to maximize functional recovery while minimizing maladaptive states after spinal cord injury.
Keywords: brain plasticity; brain-derived neurotrophic factor; exercise; pain; serotonin; spinal transection.
Copyright © 2014 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett. 2005;384:162–167. - PubMed
-
- Antri M, Mouffle C, Orsal D, Barthe JY. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur J Neurosci. 2003;18:1963–1972. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

