Inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst
- PMID: 24997360
- PMCID: PMC4404313
- DOI: 10.1016/j.ydbio.2014.06.023
Inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst
Abstract
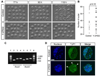
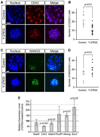
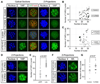
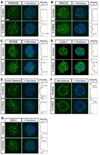
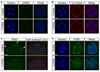
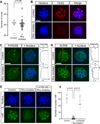
Specification of the trophectoderm (TE) and inner cell mass (ICM) lineages in the mouse blastocyst correlates with cell position, as TE derives from outer cells whereas ICM from inner cells. Differences in position are reflected by cell polarization and Hippo signaling. Only in outer cells, the apical-basal cell polarity is established, and Hippo signaling is inhibited in such a manner that LATS1 and 2 (LATS1/2) kinases are prevented from phosphorylating YAP, a key transcriptional co-activator of the TE-specifying gene Cdx2. However, the molecular mechanisms that regulate these events are not fully understood. Here, we showed that inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling and disruption of apical-basal polarity. Embryos treated with ROCK inhibitor Y-27632 exhibited elevated expression of ICM marker NANOG and reduced expression of CDX2 at the blastocyst stage. Y-27632-treated embryos failed to accumulate YAP in the nucleus, although it was rescued by concomitant inhibition of LATS1/2. Segregation between apical and basal polarity regulators, namely PARD6B, PRKCZ, SCRIB, and LLGL1, was dampened by Y-27632 treatment, whereas some of the polarization events at the late 8-cell stage such as compaction and apical localization of p-ERM and tyrosinated tubulin occurred normally. Similar abnormalities of Hippo signaling and apical-basal polarization were also observed in embryos that were treated with RHO GTPases inhibitor. These results suggest that RHO-ROCK signaling plays an essential role in regulating Hippo signaling and cell polarization to enable proper specification of the ICM and TE lineages.
Keywords: CDX2; Cell lineage; Cell polarity; Inner cell mass; Trophectoderm; YAP.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Transcription factor AP-2γ induces early Cdx2 expression and represses HIPPO signaling to specify the trophectoderm lineage.Development. 2015 May 1;142(9):1606-15. doi: 10.1242/dev.120238. Epub 2015 Apr 9. Development. 2015. PMID: 25858457 Free PMC article.
-
RHOA activity in expanding blastocysts is essential to regulate HIPPO-YAP signaling and to maintain the trophectoderm-specific gene expression program in a ROCK/actin filament-independent manner.Mol Hum Reprod. 2019 Feb 1;25(2):43-60. doi: 10.1093/molehr/gay048. Mol Hum Reprod. 2019. PMID: 30395288 Free PMC article.
-
Lineage segregation in human pre-implantation embryos is specified by YAP1 and TEAD1.Hum Reprod. 2023 Aug 1;38(8):1484-1498. doi: 10.1093/humrep/dead107. Hum Reprod. 2023. PMID: 37295962
-
A Comparative Analysis of Hippo Signaling Pathway Components during Murine and Bovine Early Mammalian Embryogenesis.Genes (Basel). 2021 Feb 16;12(2):281. doi: 10.3390/genes12020281. Genes (Basel). 2021. PMID: 33669396 Free PMC article. Review.
-
Position- and polarity-dependent Hippo signaling regulates cell fates in preimplantation mouse embryos.Semin Cell Dev Biol. 2015 Dec;47-48:80-7. doi: 10.1016/j.semcdb.2015.05.003. Epub 2015 May 15. Semin Cell Dev Biol. 2015. PMID: 25986053 Review.
Cited by
-
Transcription factor AP-2γ induces early Cdx2 expression and represses HIPPO signaling to specify the trophectoderm lineage.Development. 2015 May 1;142(9):1606-15. doi: 10.1242/dev.120238. Epub 2015 Apr 9. Development. 2015. PMID: 25858457 Free PMC article.
-
Loss of POLR1D results in embryonic lethality prior to blastocyst formation in mice.Mol Reprod Dev. 2020 Nov;87(11):1152-1158. doi: 10.1002/mrd.23427. Epub 2020 Oct 6. Mol Reprod Dev. 2020. PMID: 33022126 Free PMC article.
-
Cell signaling and transcription factors regulating cell fate during formation of the mouse blastocyst.Trends Genet. 2015 Jul;31(7):402-10. doi: 10.1016/j.tig.2015.04.002. Epub 2015 May 18. Trends Genet. 2015. PMID: 25999217 Free PMC article. Review.
-
TEAD4, YAP1 and WWTR1 prevent the premature onset of pluripotency prior to the 16-cell stage.Development. 2019 Sep 6;146(17):dev179861. doi: 10.1242/dev.179861. Development. 2019. PMID: 31444221 Free PMC article.
-
A high-content small molecule screen identifies novel inducers of definitive endoderm.Mol Metab. 2017 May 4;6(7):640-650. doi: 10.1016/j.molmet.2017.04.009. eCollection 2017 Jul. Mol Metab. 2017. PMID: 28702321 Free PMC article.
References
-
- Aktories K, Hall A. Botulinum ADP-ribosyltransferase C3: a new tool to study low molecular weight GTP-binding proteins. Trends Pharmacol. Sci. 1989;10:415–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

