The role of melanogenesis in regulation of melanoma behavior: melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways
- PMID: 24997364
- PMCID: PMC4221528
- DOI: 10.1016/j.abb.2014.06.030
The role of melanogenesis in regulation of melanoma behavior: melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways
Abstract
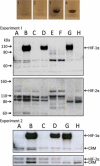
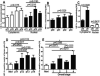
To study the effect of melanogenesis on HIF-1α expression and attendant pathways, we used stable human and hamster melanoma cell lines in which the amelanotic vs. melanotic phenotypes are dependent upon the concentration of melanogenesis precursors in the culture media. The induction of melanin pigmentation led to significant up-regulation of HIF-1α, but not HIF-2α, protein in melanized cells for both lines. Similar upregulation of nuclear HIF-1α was observed in excisions of advanced melanotic vs. amelanotic melanomas. In cultured cells, melanogenesis also significantly stimulated expression of classical HIF-1-dependent target genes involved in angiogenesis and cellular metabolism, including glucose metabolism and stimulation of activity of key enzymes in the glycolytic pathway. Several other stress related genes containing putative HRE consensus sites were also upregulated by melanogenesis, concurrently with modulation of expression of HIF-1-independent genes encoding for steroidogenic enzymes, cytokines and growth factors. Immunohistochemical studies using a large panel of pigmented lesions revealed that higher levels of HIF-1α and GLUT-1 were detected in advanced melanomas in comparison to melanocytic nevi or thin melanomas localized to the skin. However, the effects on overall or disease free survival in melanoma patients were modest or absent for GLUT-1 or for HIF-1α, respectively. In conclusion, induction of the melanogenic pathway leads to robust upregulation of HIF-1-dependent and independent pathways in cultured melanoma cells, suggesting a key role for melanogenesis in regulation of cellular metabolism.
Keywords: Glycolysis; HIF-1; Melanogenesis; Melanoma; Melanoma progression; Metabolism.
Figures




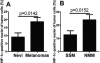

References
-
- Levine D, Fisher DE. Methods Mol Biol. 2014;1102:177–197. - PubMed
-
- Schadendorf D, Hauschild A. Nat Rev Clin Oncol. 2014 - PubMed
-
- Kwong LN, Davies MA. Oncogene. 2014;33:1–9. - PubMed
-
- Hanahan D, Weinberg RA. Cell. 2011;144:646–674. - PubMed
-
- Robert C, Dummer R, Gutzmer R, Lorigan P, Kim KB, Nyakas M, Arance A, Liszkay G, Schadendorf D, Cantarini M, Spencer S, Middleton MR. Lancet Oncol. 2013;14:733–740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

