Detection and quantification of bacterial biofilms combining high-frequency acoustic microscopy and targeted lipid microparticles
- PMID: 24997588
- PMCID: PMC4113671
- DOI: 10.1186/1477-3155-12-24
Detection and quantification of bacterial biofilms combining high-frequency acoustic microscopy and targeted lipid microparticles
Abstract
Background: Immuno-compromised patients such as those undergoing cancer chemotherapy are susceptible to bacterial infections leading to biofilm matrix formation. This surrounding biofilm matrix acts as a diffusion barrier that binds up antibiotics and antibodies, promoting resistance to treatment. Developing non-invasive imaging methods that detect biofilm matrix in the clinic are needed. The use of ultrasound in conjunction with targeted ultrasound contrast agents (UCAs) may provide detection of early stage biofilm matrix formation and facilitate optimal treatment.
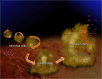
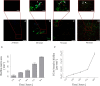
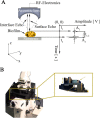
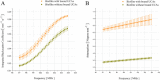
Results: Ligand-targeted UCAs were investigated as a novel method for pre-clinical non-invasive molecular imaging of early and late stage biofilms. These agents were used to target, image and detect Staphylococcus aureus biofilm matrix in vitro. Binding efficacy was assessed on biofilm matrices with respect to their increasing biomass ranging from 3.126 × 103 ± 427 UCAs per mm(2) of biofilm surface area within 12 h to 21.985 × 103 ± 855 per mm(2) of biofilm matrix surface area at 96 h. High-frequency acoustic microscopy was used to ultrasonically detect targeted UCAs bound to a biofilm matrix and to assess biofilm matrix mechanoelastic physical properties. Acoustic impedance data demonstrated that biofilm matrices exhibit impedance values (1.9 MRayl) close to human tissue (1.35 - 1.85 MRayl for soft tissues). Moreover, the acoustic signature of mature biofilm matrices were evaluated in terms of integrated backscatter (0.0278 - 0.0848 mm(-1) × sr(-1)) and acoustic attenuation (3.9 Np/mm for bound UCAs; 6.58 Np/mm for biofilm alone).
Conclusions: Early diagnosis of biofilm matrix formation is a challenge in treating cancer patients with infection-associated biofilms. We report for the first time a combined optical and acoustic evaluation of infectious biofilm matrices. We demonstrate that acoustic impedance of biofilms is similar to the impedance of human tissues, making in vivo imaging and detection of biofilm matrices difficult. The combination of ultrasound and targeted UCAs can be used to enhance biofilm imaging and early detection. Our findings suggest that the combination of targeted UCAs and ultrasound is a novel molecular imaging technique for the detection of biofilms. We show that high-frequency acoustic microscopy provides sufficient spatial resolution for quantification of biofilm mechanoelastic properties.
Figures


References
-
- Liu D, Lau YL, Chau YK, Pacepavicius G. Simple technique for estimation of biofilm accumulation. Bull Environ Contam Toxicol. 1994;53(6):913–918. - PubMed
-
- Allison DG, Ruiz B, SanJose C, Jaspe A, Gilbert P. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. Fems Microbiol Letters. 1998;167(2):179–184. - PubMed
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. - PubMed
-
- Wingender J, Neu TR, Flemming HC. In: Microbial Extracellular Polymeric Substances: Characterization, Structure and Function. 1. Wingender J, Neu TR, Flemming HC, editor. Berlin: Springer; 1999. What are Bacterial Extracellular Substances? pp. 1–19.
-
- Strathmann M, Wingender J, Flemming HC. Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of pseudomonas aeruginosa. J Microbiol Methods. 2002;50(3):237–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

