Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain
- PMID: 24998751
- PMCID: PMC4322675
- DOI: 10.1016/j.neuropharm.2014.06.022
Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain
Abstract
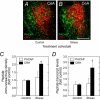
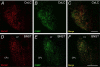
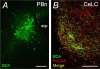
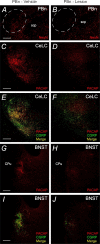
The intricate relationships that associate pain, stress responses and emotional behavior have been well established. Acute stressful situations can decrease nociceptive sensations and conversely, chronic pain can enhance other pain experiences and heighten the emotional and behavioral consequences of stress. Accordingly, chronic pain is comorbid with a number of behavioral disorders including depression, anxiety abnormalities and associated stress-related disorders including post traumatic stress disorder (PTSD). The central nucleus of the amygdala (CeA) represents a convergence of pathways for pain, stress and emotion, and we have identified pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in fiber elements in the lateral capsular division of the CeA (CeLC). The PACAP staining patterns colocalized in part with those for calcitonin gene related peptide (CGRP); anterograde fiber tracing and excitotoxic lesion studies demonstrated that the CeLC PACAP/CGRP immunoreactivities represented sensory fiber projections from the lateral parabrachial nucleus (LPBn) along the spino-parabrachioamygdaloid tract. The same PBn PACAP/CGRP fiber system also projected to the BNST. As in the BNST, CeA PACAP signaling increased anxiety-like behaviors accompanied by weight loss and decreased feeding. But in addition to heightened anxiety-like responses, CeA PACAP signaling also altered nociception as reflected by decreased latency and threshold responses in thermal and mechanical sensitivity tests, respectively. From PACAP expression in major pain pathways, the current observations are novel and suggest that CeA PACAP nociceptive signaling and resulting neuroplasticity via the spino-parabrachioamygdaloid tract may represent mechanisms that associate chronic pain with sensory hypersensitivity, fear memory consolidation and severe behavioral disorders.
Keywords: CGRP; Central amygdala; Hypersensitivity; Lateral capsular division; PACAP; Parabrachial nucleus; Parabrachialamygdaloid tract.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

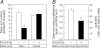
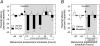
Similar articles
-
Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies.Biol Psychiatry. 2015 Aug 1;78(3):167-77. doi: 10.1016/j.biopsych.2014.12.003. Epub 2014 Dec 9. Biol Psychiatry. 2015. PMID: 25636177 Free PMC article. Review.
-
Parabrachial Pituitary Adenylate Cyclase-Activating Polypeptide Activation of Amygdala Endosomal Extracellular Signal-Regulated Kinase Signaling Regulates the Emotional Component of Pain.Biol Psychiatry. 2017 Apr 15;81(8):671-682. doi: 10.1016/j.biopsych.2016.08.025. Epub 2016 Aug 29. Biol Psychiatry. 2017. PMID: 28057459 Free PMC article.
-
Activation of Lateral Parabrachial Nucleus (LPBn) PACAP-Expressing Projection Neurons to the Bed Nucleus of the Stria Terminalis (BNST) Enhances Anxiety-like Behavior.J Mol Neurosci. 2022 Mar;72(3):451-458. doi: 10.1007/s12031-021-01946-z. Epub 2021 Nov 22. J Mol Neurosci. 2022. PMID: 34811712 Free PMC article.
-
Stimulation of lateral parabrachial (LPB) to central amygdala (CeA) pituitary adenylate cyclase-activating polypeptide (PACAP) neurons induces anxiety-like behavior and mechanical allodynia.Pharmacol Biochem Behav. 2023 Sep;230:173605. doi: 10.1016/j.pbb.2023.173605. Epub 2023 Jul 25. Pharmacol Biochem Behav. 2023. PMID: 37499765
-
Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress.J Mol Neurosci. 2010 Nov;42(3):327-40. doi: 10.1007/s12031-010-9364-7. Epub 2010 Apr 20. J Mol Neurosci. 2010. PMID: 20405238 Free PMC article. Review.
Cited by
-
Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies.Biol Psychiatry. 2015 Aug 1;78(3):167-77. doi: 10.1016/j.biopsych.2014.12.003. Epub 2014 Dec 9. Biol Psychiatry. 2015. PMID: 25636177 Free PMC article. Review.
-
Fear extinction learning ability predicts neuropathic pain behaviors and amygdala activity in male rats.Mol Pain. 2018 Jan-Dec;14:1744806918804441. doi: 10.1177/1744806918804441. Epub 2018 Sep 13. Mol Pain. 2018. PMID: 30209982 Free PMC article.
-
The Paraventricular Hypothalamus Regulates Satiety and Prevents Obesity via Two Genetically Distinct Circuits.Neuron. 2019 May 8;102(3):653-667.e6. doi: 10.1016/j.neuron.2019.02.028. Epub 2019 Mar 14. Neuron. 2019. PMID: 30879785 Free PMC article.
-
Exposure to hot and cold environments increases noradrenaline release in the bed nucleus of the stria terminalis in rats.Neuropsychopharmacol Rep. 2018 Dec;38(4):214-218. doi: 10.1002/npr2.12036. Epub 2018 Oct 19. Neuropsychopharmacol Rep. 2018. PMID: 30341818 Free PMC article.
-
The PACAP/PAC1 Receptor System and Feeding.Brain Sci. 2021 Dec 23;12(1):13. doi: 10.3390/brainsci12010013. Brain Sci. 2021. PMID: 35053757 Free PMC article. Review.
References
-
- Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety. 2009;26:888–901. - PubMed
-
- Beaudet MM, Braas KM, May V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol. 1998;36:325–336. - PubMed
-
- Bernard JF, Bester H, Besson JM. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog Brain Res. 1996;107:243–255. - PubMed
-
- Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68:551–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-060481/DK/NIDDK NIH HHS/United States
- MH-096764/MH/NIMH NIH HHS/United States
- MH-97988/MH/NIMH NIH HHS/United States
- R56 DK060481/DK/NIDDK NIH HHS/United States
- P30 GM103498/GM/NIGMS NIH HHS/United States
- R29 DK051369/DK/NIDDK NIH HHS/United States
- R01 MH096764/MH/NIMH NIH HHS/United States
- R01 DK060481/DK/NIDDK NIH HHS/United States
- MH-080935/MH/NIMH NIH HHS/United States
- R01 MH097988/MH/NIMH NIH HHS/United States
- F32 MH072088/MH/NIMH NIH HHS/United States
- R01 DK051369/DK/NIDDK NIH HHS/United States
- R01 DK065989/DK/NIDDK NIH HHS/United States
- P30RR032135/RR/NCRR NIH HHS/United States
- R21 MH080935/MH/NIMH NIH HHS/United States
- DK-051369/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

