Expression of Ser729 phosphorylated PKCepsilon in experimental crescentic glomerulonephritis: an immunohistochemical study
- PMID: 24998921
- PMCID: PMC4083321
- DOI: 10.4081/ejh.2014.2308
Expression of Ser729 phosphorylated PKCepsilon in experimental crescentic glomerulonephritis: an immunohistochemical study
Abstract
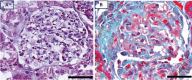
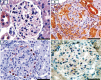
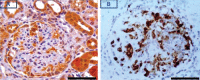
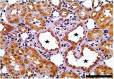
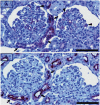
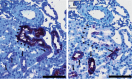
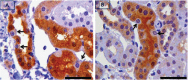
PKCε, a DAG-dependent, Ca2+- independent kinase attenuates extent of fibrosis following tissue injury, suppresses apoptosis and promotes cell quiescence. In crescentic glomerulonephritis (CGN), glomerular epithelial cells (GEC) contribute to fibro-cellular crescent formation while they also transdifferentiate to a mesenchymal phenotype. The aim of this study was to assess PKCε expression in CGN. Using an antibody against PKC-ε phosphorylated at Ser729, we assessed its localization in rat model of immune-mediated rapidly progressive CGN. In glomeruli of control animals, pPKCε was undetectable. In animals with CGN, pPKCε was expressed exclusively in glomerular epithelial cells (GEC) and in GEC comprising fibrocellular crescents that had acquired a myofibroblast-type phenotype. In non-immune GEC injury induced by puromycin aminonucleoside and resulting in proteinuria of similar magnitude as in CGN, pPKCε expression was absent. There was constitutive pPKCε expression in distal convoluted tubules, collecting ducts and thick segments of Henley's loops in both control and experimental animals. We propose that pPKCε expression occurring in GEC and in fibrocellular crescentic lesions in CGN may facilitate PKCε dependent pathologic processes.
Figures

Similar articles
-
Glomerular expression of C-C chemokines in different types of human crescentic glomerulonephritis.Nephrol Dial Transplant. 2003 Aug;18(8):1526-34. doi: 10.1093/ndt/gfg172. Nephrol Dial Transplant. 2003. PMID: 12897090
-
GEC-targeted HO-1 expression reduces proteinuria in glomerular immune injury.Am J Physiol Renal Physiol. 2009 Sep;297(3):F629-38. doi: 10.1152/ajprenal.00213.2009. Epub 2009 Jul 8. Am J Physiol Renal Physiol. 2009. PMID: 19587144 Free PMC article.
-
Role of integrin-linked kinase in epithelial-mesenchymal transition in crescent formation of experimental glomerulonephritis.Nephrol Dial Transplant. 2006 Sep;21(9):2380-90. doi: 10.1093/ndt/gfl243. Epub 2006 May 25. Nephrol Dial Transplant. 2006. PMID: 16728424
-
The glomerular crescent: triggers, evolution, resolution, and implications for therapy.Curr Opin Nephrol Hypertens. 2020 May;29(3):302-309. doi: 10.1097/MNH.0000000000000596. Curr Opin Nephrol Hypertens. 2020. PMID: 32132388 Free PMC article. Review.
-
Parietal epithelial cell dysfunction in crescentic glomerulonephritis.Cell Tissue Res. 2021 Aug;385(2):345-354. doi: 10.1007/s00441-021-03513-9. Epub 2021 Aug 28. Cell Tissue Res. 2021. PMID: 34453566 Free PMC article. Review.
Cited by
-
Histochemistry in biology and medicine: a message from the citing journals.Eur J Histochem. 2015 Dec 23;59(4):2610. doi: 10.4081/ejh.2015.2610. Eur J Histochem. 2015. PMID: 26708189 Free PMC article. Review.
-
Role of FGFR2c and Its PKCε Downstream Signaling in the Control of EMT and Autophagy in Pancreatic Ductal Adenocarcinoma Cells.Cancers (Basel). 2021 Oct 5;13(19):4993. doi: 10.3390/cancers13194993. Cancers (Basel). 2021. PMID: 34638477 Free PMC article.
-
Impact of Histochemistry on biomedical research: looking through the articles published in a long-established histochemical journal.Eur J Histochem. 2014 Dec 30;58(4):2474. doi: 10.4081/ejh.2014.2474. Eur J Histochem. 2014. PMID: 25578981 Free PMC article.
-
The FGFR2c/PKCε Axis Controls MCL-1-Mediated Invasion in Pancreatic Ductal Adenocarcinoma Cells: Perspectives for Innovative Target Therapies.Biomedicines. 2022 Jul 9;10(7):1652. doi: 10.3390/biomedicines10071652. Biomedicines. 2022. PMID: 35884957 Free PMC article.
-
Role of PKCε in the epithelial-mesenchymal transition induced by FGFR2 isoform switch.Cell Commun Signal. 2020 May 19;18(1):76. doi: 10.1186/s12964-020-00582-1. Cell Commun Signal. 2020. PMID: 32429937 Free PMC article.
References
-
- Pfaff IL, Wagner HJ, Vallon V. Immunolocalization of protein kinase C isoenzymes alpha, beta1 and betaII in rat kidney. J Am Soc Nephrol 1999;10:1861-73 - PubMed
-
- Tipping PG. Crescentic nephritis--is it in your genes? Nephrol Dial Transplant 2008;23:3065-6 - PubMed
-
- Cattell V. Macrophages in acute glomerular inflammation. Kidney Int 1994;45:945-52 - PubMed
-
- Ganz MB, Abu-Nader R, Saxena R, Grond J. Protein kinase C beta II isoform is up-regulated in human proliferative glomerulonephritis. Exp Nephrol 1997;5:225-32 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

