Management of long-term and reversible hysteroscopic sterilization: a novel device with nickel-titanium shape memory alloy
- PMID: 24999021
- PMCID: PMC4105153
- DOI: 10.1186/1477-7827-12-61
Management of long-term and reversible hysteroscopic sterilization: a novel device with nickel-titanium shape memory alloy
Abstract
Background: Female sterilization is the second most commonly used method of contraception in the United States. Female sterilization can now be performed through laparoscopic, abdominal, or hysteroscopic approaches. The hysteroscopic sterilization may be a safer option than sterilization through laparoscopy or laparotomy because it avoids invading the abdominal cavity and undergoing general anaesthesia. Hysteroscopic sterilization mainly includes chemical agents and mechanical devices. Common issues related to the toxicity of the chemical agents used have raised concerns regarding this kind of contraception. The difficulty of the transcervical insertion of such mechanical devices into the fallopian tubes has increased the high incidence of device displacement or dislodgment. At present, Essure® is the only commercially available hysteroscopic sterilization device being used clinically. The system is irreversible and is not effective immediately.
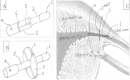
Presentation of the hypothesis: Our new hysteroscopic sterility system consists of nickel-titanium (NiTi) shape memory alloy and a waterproof membrane. The NiTi alloy is covered with two coatings to avoid toxic Ni release and to prevent stimulation of epithelial tissue growth around the oviducts. Because of the shape memory effect of the NiTi alloy, the device works like an umbrella: it stays collapsed at low temperature before placement and opens by the force of shape memory activated by the body temperature after it is inserted hysteroscopically into the interstitial tubal lumen. The rim of the open device will incise into interstitial myometrium during the process of unfolding. Once the device is fixed, it blocks the tube completely. When the patient no longer wishes for sterilization, the device can be closed by perfusing liquid with low temperature into the uterine cavity, followed by prospective hysteroscopic removal. After the device removal, the fallopian tube will revert to its physiological functions.
Testing the hypothesis: Currently, experimental and clinical studies are needed to attest the safety, efficiency and reversibility of the novel sterilization device.
Implications of the hypothesis: If our hypothesis is confirmed, appropriate and reversible contraceptive can be achieved with the device we have designed, which may have significant repercussions for numerous women worldwide.
Figures
References
-
- Castaño PM, Adekunle L. Transcervical sterilization. Semin Reprod Med. 2010;28:103–109. - PubMed
-
- Abbott J. Transcervical sterilization. Curr Opin Obstet Gynecol. 2007;19:325–330. - PubMed
-
- Smith RD. Contemporary hysteroscopic methods for female sterilization. Int J Gynaecol Obstet. 2010;108:79–84. - PubMed
-
- Kraemer DF, Yen PY, Nichols M. An economic comparison of female sterilization of hysteroscopic tubal occlusion with laparoscopic bilateral tubal ligation. Contraception. 2009;80:254–260. - PubMed
-
- Maubon AJ, Thurmond AS, Laurent A, Honiger JE, Scanlan RM, Rouanet JP. Selective tubal sterilization in rabbits: experience with a hydrogel combined with a sclerosing agent. Radiology. 1994;193:721–723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

