Measurement of neutralizing serum antibodies of patients vaccinated with human papillomavirus L1 or L2-based immunogens using furin-cleaved HPV Pseudovirions
- PMID: 24999962
- PMCID: PMC4084990
- DOI: 10.1371/journal.pone.0101576
Measurement of neutralizing serum antibodies of patients vaccinated with human papillomavirus L1 or L2-based immunogens using furin-cleaved HPV Pseudovirions
Abstract
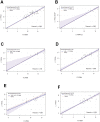
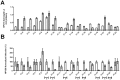
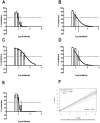
Antibodies specific for neutralizing epitopes in either Human papillomavirus (HPV) capsid protein L1 or L2 can mediate protection from viral challenge and thus their accurate and sensitive measurement at high throughput is likely informative for monitoring response to prophylactic vaccination. Here we compare measurement of L1 and L2-specific neutralizing antibodies in human sera using the standard Pseudovirion-Based Neutralization Assay (L1-PBNA) with the newer Furin-Cleaved Pseudovirion-Based Neutralization Assay (FC-PBNA), a modification of the L1-PBNA intended to improve sensitivity towards L2-specific neutralizing antibodies without compromising assay of L1-specific responses. For detection of L1-specific neutralizing antibodies in human sera, the FC- PBNA and L1-PBNA assays showed similar sensitivity and a high level of correlation using WHO standard sera (n = 2), and sera from patients vaccinated with Gardasil (n = 30) or an experimental human papillomavirus type 16 (HPV16) L1 VLP vaccine (n = 70). The detection of L1-specific cross-neutralizing antibodies in these sera using pseudovirions of types phylogenetically-related to those targeted by the L1 virus-like particle (VLP) vaccines was also consistent between the two assays. However, for sera from patients (n = 17) vaccinated with an L2-based immunogen (TA-CIN), the FC-PBNA was more sensitive than the L1-PBNA in detecting L2-specific neutralizing antibodies. Further, the neutralizing antibody titers measured with the FC-PBNA correlated with those determined with the L2-PBNA, another modification of the L1-PBNA that spacio-temporally separates primary and secondary receptor engagement, as well as the protective titers measured using passive transfer studies in the murine genital-challenge model. In sum, the FC-PBNA provided sensitive measurement for both L1 VLP and L2-specific neutralizing antibody in human sera. Vaccination with TA-CIN elicits weak cross-protective antibody in a subset of patients, suggesting the need for an adjuvant.
Conflict of interest statement
Figures
Similar articles
-
Production of Furin-Cleaved Papillomavirus Pseudovirions and Their Use for In Vitro Neutralization Assays of L1- or L2-Specific Antibodies.Curr Protoc Microbiol. 2015 Aug 3;38:14B.5.1-26. doi: 10.1002/9780471729259.mc14b05s38. Curr Protoc Microbiol. 2015. PMID: 26237105 Free PMC article.
-
Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines.J Natl Cancer Inst. 2009 Jun 3;101(11):782-92. doi: 10.1093/jnci/djp106. Epub 2009 May 26. J Natl Cancer Inst. 2009. PMID: 19470949 Free PMC article.
-
Multivalent human papillomavirus l1 DNA vaccination utilizing electroporation.PLoS One. 2013;8(3):e60507. doi: 10.1371/journal.pone.0060507. Epub 2013 Mar 25. PLoS One. 2013. PMID: 23536912 Free PMC article.
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
-
Papillomavirus-like particle vaccines.J Natl Cancer Inst Monogr. 2001;(28):50-4. doi: 10.1093/oxfordjournals.jncimonographs.a024258. J Natl Cancer Inst Monogr. 2001. PMID: 11158207 Review.
Cited by
-
Production of Furin-Cleaved Papillomavirus Pseudovirions and Their Use for In Vitro Neutralization Assays of L1- or L2-Specific Antibodies.Curr Protoc Microbiol. 2015 Aug 3;38:14B.5.1-26. doi: 10.1002/9780471729259.mc14b05s38. Curr Protoc Microbiol. 2015. PMID: 26237105 Free PMC article.
-
Roles of Fc Domain and Exudation in L2 Antibody-Mediated Protection against Human Papillomavirus.J Virol. 2018 Jul 17;92(15):e00572-18. doi: 10.1128/JVI.00572-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29743371 Free PMC article.
-
Vaccination with a Human Papillomavirus L2 Multimer Provides Broad Protection against 17 Human Papillomavirus Types in the Mouse Cervicovaginal Challenge Model.Vaccines (Basel). 2024 Jun 20;12(6):689. doi: 10.3390/vaccines12060689. Vaccines (Basel). 2024. PMID: 38932417 Free PMC article.
-
Seroepidemiology of Human Papillomavirus 16 (HPV16) L2 and Generation of L2-Specific Human Chimeric Monoclonal Antibodies.Clin Vaccine Immunol. 2015 Jul;22(7):806-16. doi: 10.1128/CVI.00799-14. Epub 2015 May 13. Clin Vaccine Immunol. 2015. PMID: 25972404 Free PMC article.
-
Durable immunity to oncogenic human papillomaviruses elicited by adjuvanted recombinant Adeno-associated virus-like particle immunogen displaying L2 17-36 epitopes.Vaccine. 2015 Oct 13;33(42):5553-5563. doi: 10.1016/j.vaccine.2015.09.005. Epub 2015 Sep 15. Vaccine. 2015. PMID: 26382603 Free PMC article.
References
-
- Schiller JT, Lowy DR (2014) Virus infection and human cancer: an overview. Recent Results Cancer Res 193: 1–10. - PubMed
-
- Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, et al. (2001) Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 93: 284–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

