Cross-talk-free multi-color STORM imaging using a single fluorophore
- PMID: 25000286
- PMCID: PMC4084994
- DOI: 10.1371/journal.pone.0101772
Cross-talk-free multi-color STORM imaging using a single fluorophore
Erratum in
- PLoS One. 2014;9(10):e111878
Abstract
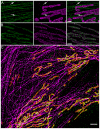
Multi-color stochastic optical reconstruction microscopy (STORM) is routinely performed; however, the various approaches for achieving multiple colors have important caveats. Color cross-talk, limited availability of spectrally distinct fluorophores with optimal brightness and duty cycle, incompatibility of imaging buffers for different fluorophores, and chromatic aberrations impact the spatial resolution and ultimately the number of colors that can be achieved. We overcome these complexities and develop a simple approach for multi-color STORM imaging using a single fluorophore and sequential labelling. In addition, we present a simple and versatile method to locate the same region of interest on different days and even on different microscopes. In combination, these approaches enable cross-talk-free multi-color imaging of sub-cellular structures.
Conflict of interest statement
Figures



References
-
- Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, et al. (2006) Imaging intracellular fluorescent proteins at nanometer resolution. Science 313: 1642–1645. - PubMed
-
- Yildiz A, Selvin PR (2005) Fluorescence imaging with one nanometer accuracy: application to molecular motors. Acc Chem Res 38: 574–582. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

