Laser-supported CD133+ cell therapy in patients with ischemic cardiomyopathy: initial results from a prospective phase I multicenter trial
- PMID: 25000346
- PMCID: PMC4084817
- DOI: 10.1371/journal.pone.0101449
Laser-supported CD133+ cell therapy in patients with ischemic cardiomyopathy: initial results from a prospective phase I multicenter trial
Erratum in
- PLoS One. 2014;9(8):e107082
Abstract
Objectives: This study evaluates the safety, principal feasibility and restoration potential of laser-supported CD133+ intramyocardial cell transplantation in patients with ischemic cardiomyopathy.
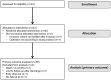
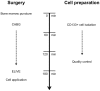
Methods: Forty-two patients with severe ischemic cardiomyopathy (left ventricular ejection fraction (LVEF) >15% and <35%) were included in this prospective multicenter phase I trial. They underwent coronary artery bypass grafting (CABG) with subsequent transepicardial low-energy laser treatment and autologous CD133+ cell transplantation, and were followed up for 12 months. To evaluate segmental myocardial contractility as well as perfusion and to identify the areas of scar tissue, cardiac MRI was performed at 6 months and compared to the preoperative baseline. In addition, clinical assessment comprising of CCS scoring, blood and physical examination was performed at 3, 6 and 12 months, respectively.
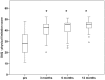
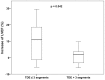
Results: Intraoperative cell isolation resulted in a mean cell count of 9.7±1.2×106. Laser treatment and subsequent CD133+ cell therapy were successfully and safely carried out in all patients and no procedure-related complications occurred. At 6 months, the LVEF was significantly increased (29.7±1.9% versus 24.6±1.5% with p = 0.004). In addition, freedom from angina was achieved, and quality of life significantly improved after therapy (p<0.0001). Interestingly, an extended area of transmural delayed enhancement (>3 myocardial segments) determined in the preoperative MRI was inversely correlated with a LVEF increase after laser-supported cell therapy (p = 0.024).
Conclusions: This multicenter trial demonstrates that laser-supported CD133+ cell transplantation is safe and feasible in patients with ischemic cardiomyopathy undergoing CABG, and in most cases, it appears to significantly improve the myocardial function. Importantly, our data show that the beneficial effect was significantly related to the extent of transmural delayed enhancement, suggesting that MRI-guided selection of patients is mandatory to ensure the effectiveness of the therapy.
Trial registration: EudraCT 2005-004051-35) Controlled-Trials.com ISRCTN49998633.
Conflict of interest statement
Figures

References
-
- Klein HM, Ghodsizad A, Marktanner R, Poll L, Voelkel T, et al. (2007) Intramyocardial implantation of CD133+ stem cells improved cardiac function without bypass surgery. Heart Surg Forum 10: E66–E69. - PubMed
-
- Menasché P (2008) Cardiac cell therapy trials: chronic myocardial infarction and congestive heart failure. J Cardiovasc Transl Res 1: 201–206. - PubMed
-
- Manginas A, Goussetis E, Koutelou M, Karatasakis G, Peristeri I, et al. (2007) Pilot study to evaluate the safety and feasibility of intracoronary CD133(+) and CD133(−) CD34(+) cell therapy in patients with nonviable anterior myocardial infarction. Catheter Cardiovasc Interv 69: 773–781. - PubMed
-
- Strauer BE, Steinhoff G (2011) 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J Am Coll Cardiol 58: 1095–1104. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

