Genome-wide analysis captures the determinants of the antibiotic cross-resistance interaction network
- PMID: 25000950
- PMCID: PMC4102323
- DOI: 10.1038/ncomms5352
Genome-wide analysis captures the determinants of the antibiotic cross-resistance interaction network
Abstract
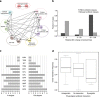
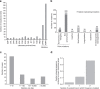
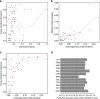
Understanding how evolution of antimicrobial resistance increases resistance to other drugs is a challenge of profound importance. By combining experimental evolution and genome sequencing of 63 laboratory-evolved lines, we charted a map of cross-resistance interactions between antibiotics in Escherichia coli, and explored the driving evolutionary principles. Here, we show that (1) convergent molecular evolution is prevalent across antibiotic treatments, (2) resistance conferring mutations simultaneously enhance sensitivity to many other drugs and (3) 27% of the accumulated mutations generate proteins with compromised activities, suggesting that antibiotic adaptation can partly be achieved without gain of novel function. By using knowledge on antibiotic properties, we examined the determinants of cross-resistance and identified chemogenomic profile similarity between antibiotics as the strongest predictor. In contrast, cross-resistance between two antibiotics is independent of whether they show synergistic effects in combination. These results have important implications on the development of novel antimicrobial strategies.
Figures

References
-
- Cooper V. S. & Lenski R. E. The population genetics of ecological specialization in evolving Escherichia coli populations. Nature 407, 736–739 (2000). - PubMed
-
- Futuyma D. J. & Moreno G. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 19, 207–233 (1988).
Publication types
MeSH terms
Associated data
- BioProject/PRJNA248327
- SRA/SRP042209
- SRA/SRR1297006
- SRA/SRR1297043
- SRA/SRR1297049
- SRA/SRR1297054
- SRA/SRR1297055
- SRA/SRR1297056
- SRA/SRR1297060
- SRA/SRR1297061
- SRA/SRR1297062
- SRA/SRR1297063
- SRA/SRR1297064
- SRA/SRR1297067
- SRA/SRR1297069
- SRA/SRR1297073
- SRA/SRR1297077
- SRA/SRR1297079
- SRA/SRR1297081
- SRA/SRR1297096
- SRA/SRR1297101
- SRA/SRR1297103
- SRA/SRR1297104
- SRA/SRR1297105
- SRA/SRR1297106
- SRA/SRR1297107
- SRA/SRR1297109
- SRA/SRR1297112
- SRA/SRR1297114
- SRA/SRR1297117
- SRA/SRR1297123
- SRA/SRR1297124
- SRA/SRR1297125
- SRA/SRR1297126
- SRA/SRR1297129
- SRA/SRR1297132
- SRA/SRR1297133
- SRA/SRR1297135
- SRA/SRR1297137
- SRA/SRR1297139
- SRA/SRR1297142
- SRA/SRR1297144
- SRA/SRR1297148
- SRA/SRR1297150
- SRA/SRR1297153
- SRA/SRR1297155
- SRA/SRR1297157
- SRA/SRR1297159
- SRA/SRR1297160
- SRA/SRR1297163
- SRA/SRR1297168
- SRA/SRR1297169
- SRA/SRR1297170
- SRA/SRR1297171
- SRA/SRR1297172
- SRA/SRR1297173
- SRA/SRR1297175
- SRA/SRR1297176
- SRA/SRR1297177
- SRA/SRR1297178
- SRA/SRR1297179
- SRA/SRR1297180
- SRA/SRR1297181
- SRA/SRR1297182
- SRA/SRR1297183
- SRA/SRR1297184
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

