Distinct genetic programs guide Drosophila circular and longitudinal visceral myoblast fusion
- PMID: 25000973
- PMCID: PMC4169254
- DOI: 10.1186/1471-2121-15-27
Distinct genetic programs guide Drosophila circular and longitudinal visceral myoblast fusion
Abstract
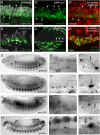
Background: The visceral musculature of Drosophila larvae comprises circular visceral muscles tightly interwoven with longitudinal visceral muscles. During myogenesis, the circular muscles arise by one-to-one fusion of a circular visceral founder cell (FC) with a visceral fusion-competent myoblast (FCM) from the trunk visceral mesoderm, and longitudinal muscles arise from FCs of the caudal visceral mesoderm. Longitudinal FCs migrate anteriorly under guidance of fibroblast growth factors during embryogenesis; it is proposed that they fuse with FCMs from the trunk visceral mesoderm to give rise to syncytia containing up to six nuclei.
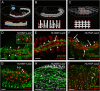
Results: Using fluorescence in situ hybridization and immunochemical analyses, we investigated whether these fusion events during migration use the same molecular repertoire and cellular components as fusion-restricted myogenic adhesive structure (FuRMAS), the adhesive signaling center that mediates myoblast fusion in the somatic mesoderm. Longitudinal muscles were formed by the fusion of one FC with Sns-positive FCMs, and defects in FCM specification led to defects in longitudinal muscle formation. At the fusion sites, Duf/Kirre and the adaptor protein Rols7 accumulated in longitudinal FCs, and Blow and F-actin accumulated in FCMs. The accumulation of these four proteins at the fusion sites argues for FuRMAS-like adhesion and signaling centers. Longitudinal fusion was disturbed in rols and blow single, and scar wip double mutants. Mutants of wasp or its interaction partner wip had no defects in longitudinal fusion.
Conclusions: Our results indicated that all embryonic fusion events depend on the same cell-adhesion molecules, but that the need for Rols7 and regulators of F-actin distinctly differs. Rols7 was required for longitudinal visceral and somatic myoblast fusion but not for circular visceral fusion. Importantly, longitudinal fusion depended on Kette and SCAR/Wave but was independent of WASp-dependent Arp2/3 activation. Thus, the complexity of the players involved in muscle formation increases from binucleated circular muscles to longitudinal visceral muscles to somatic muscles.
Figures





References
-
- Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. Berlin: Springer-Verlag; 1985.
-
- Kusch T, Reuter R. Functions for Drosophila brachyenteron and forkhead in mesoderm specification and cell signalling. Development. 1999;126:3991–4003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

