Increased antitumor effects using IL-2 with anti-TGF-β reveals competition between mouse NK and CD8 T cells
- PMID: 25000978
- PMCID: PMC4241855
- DOI: 10.4049/jimmunol.1400034
Increased antitumor effects using IL-2 with anti-TGF-β reveals competition between mouse NK and CD8 T cells
Abstract
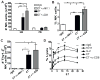
Because of increasing interest in the removal of immunosuppressive pathways in cancer, the combination of IL-2 with Abs to neutralize TGF-β, a potent immunosuppressive cytokine, was assessed. Combination immunotherapy resulted in significantly greater antitumor effects. These were correlated with significant increases in the numbers and functionality of NK cells, NK cell progenitors, and activated CD8 T cells, resulting in the observed antitumor effects. Combination immunotherapy also was accompanied by lesser toxicities than was IL-2 therapy alone. Additionally, we observed a dual competition between NK cells and activated CD8 T cells such that, after immunotherapy, the depletion of either effector population resulted in the increased total expansion of the other population and compensatory antitumor effects. This study demonstrates the efficacy of this combination immunotherapeutic regimen as a promising cancer therapy and illustrates the existence of potent competitive regulatory pathways between NK cells and CD8 T cells in response to systemic activation.
Copyright © 2014 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors disclose no conflict of interest.
Figures
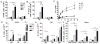
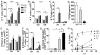

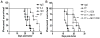

References
-
- Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. Journal of internal medicine. 2009;266:154–181. - PubMed
-
- Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. Jama. 1994;271:907–913. - PubMed
-
- Buyse M, Squifflet P, Lange BJ, Alonzo TA, Larson RA, Kolitz JE, George SL, Bloomfield CD, Castaigne S, Chevret S, Blaise D, Maraninchi D, Lucchesi KJ, Burzykowski T. Individual patient data meta-analysis of randomized trials evaluating IL-2 monotherapy as remission maintenance therapy in acute myeloid leukemia. Blood. 2011;117:7007–7013. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

