Mechanical signaling in reproductive tissues: mechanisms and importance
- PMID: 25001021
- PMCID: PMC4212346
- DOI: 10.1177/1933719114542023
Mechanical signaling in reproductive tissues: mechanisms and importance
Abstract
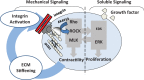
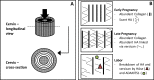
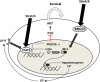
The organs of the female reproductive system are among the most dynamic tissues in the human body, undergoing repeated cycles of growth and involution from puberty through menopause. To achieve such impressive plasticity, reproductive tissues must respond not only to soluble signals (hormones, growth factors, and cytokines) but also to physical cues (mechanical forces and osmotic stress) as well. Here, we review the mechanisms underlying the process of mechanotransduction-how signals are conveyed from the extracellular matrix that surrounds the cells of reproductive tissues to the downstream molecules and signaling pathways that coordinate the cellular adaptive response to external forces. Our objective was to examine how mechanical forces contribute significantly to physiological functions and pathogenesis in reproductive tissues. We highlight how widespread diseases of the reproductive tract, from preterm labor to tumors of the uterus and breast, result from an impairment in mechanical signaling.
Keywords: ROCK/Rho kinase; cervical insufficiency; endometriosis; extracellular matrix; fibroids; mechanotransduction.
© The Author(s) 2014.
Conflict of interest statement
Figures


Similar articles
-
Dynamic reciprocity between cells and their microenvironment in reproduction.Biol Reprod. 2015 Jan;92(1):25. doi: 10.1095/biolreprod.114.121368. Epub 2014 Nov 19. Biol Reprod. 2015. PMID: 25411389 Free PMC article. Review.
-
Mechanosensing and mechanochemical transduction: how is mechanical energy sensed and converted into chemical energy in an extracellular matrix?Crit Rev Biomed Eng. 2003;31(4):255-331. doi: 10.1615/critrevbiomedeng.v31.i4.10. Crit Rev Biomed Eng. 2003. PMID: 15095950 Review.
-
Relaxin physiology in the female reproductive tract during pregnancy.Adv Exp Med Biol. 2007;612:34-48. doi: 10.1007/978-0-387-74672-2_4. Adv Exp Med Biol. 2007. PMID: 18161480 Review.
-
Cell response to mechanical microenvironment cues via Rho signaling: From mechanobiology to mechanomedicine.Acta Biomater. 2023 Mar 15;159:1-20. doi: 10.1016/j.actbio.2023.01.039. Epub 2023 Jan 28. Acta Biomater. 2023. PMID: 36717048 Review.
-
Cervical collagen network remodeling in normal pregnancy and disrupted parturition in Antxr2 deficient mice.J Biomech Eng. 2014 Feb;136(2):021017. doi: 10.1115/1.4026423. J Biomech Eng. 2014. PMID: 24390076 Free PMC article.
Cited by
-
Early menarche and childbirth accelerate aging-related outcomes and age-related diseases: Evidence for antagonistic pleiotropy in humans.Elife. 2025 Aug 12;13:RP102447. doi: 10.7554/eLife.102447. Elife. 2025. PMID: 40792619 Free PMC article.
-
Biomechanics and mechanical signaling in the ovary: a systematic review.J Assist Reprod Genet. 2018 Jul;35(7):1135-1148. doi: 10.1007/s10815-018-1180-y. Epub 2018 Apr 24. J Assist Reprod Genet. 2018. PMID: 29691711 Free PMC article.
-
Endometrium On-a-Chip Reveals Insulin- and Glucose-induced Alterations in the Transcriptome and Proteomic Secretome.Endocrinology. 2021 Jun 1;162(6):bqab054. doi: 10.1210/endocr/bqab054. Endocrinology. 2021. PMID: 33693651 Free PMC article.
-
The 300 versus 300 Study-Low Volume versus High Volume Single Balloon Catheter for Induction of Labor: A Retrospective Case-Control Study.J Clin Med. 2023 Jul 22;12(14):4839. doi: 10.3390/jcm12144839. J Clin Med. 2023. PMID: 37510954 Free PMC article.
-
Lysyl Oxidases Are Necessary for Myometrial Contractility and On-time Parturition in Mice.J Endocr Soc. 2025 Feb 14;9(5):bvaf028. doi: 10.1210/jendso/bvaf028. eCollection 2025 May. J Endocr Soc. 2025. PMID: 40170697 Free PMC article.
References
-
- Bissel MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99 (1):31–68 - PubMed
-
- Ingber DE. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3 (5):841–848 - PubMed
-
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20 (7):811–827 - PubMed
-
- Sukharev S, Corey D. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci STKE. 2004;2004(219):re4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

