Comparative nonclinical assessments of the proposed biosimilar PF-05280014 and trastuzumab (Herceptin(®))
- PMID: 25001079
- PMCID: PMC4176567
- DOI: 10.1007/s40259-014-0103-4
Comparative nonclinical assessments of the proposed biosimilar PF-05280014 and trastuzumab (Herceptin(®))
Abstract
Background and objectives: Trastuzumab (Herceptin(®)) is a humanized monoclonal antibody (mAb) that binds to the HER2 protein. PF-05280014 is being developed as a potential biosimilar to trastuzumab products marketed in the United States (trastuzumab-US) and European Union (trastuzumab-EU). Nonclinical studies were designed to evaluate the similarity of PF-05280014 to trastuzumab-US and trastuzumab-EU using in vitro structural and functional analyses, and in vivo pharmacokinetic and immunogenicity assessments.
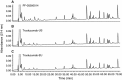
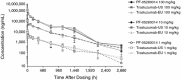
Methods: Peptide mapping was utilized to determine structural similarity. Functional similarity was assessed via an in vitro tumor cell growth inhibition assay. CD-1 male mice were administered a single-dose (0, 1, 10, or 100 mg/kg) of PF-05280014, trastuzumab-US, or trastuzumab-EU. Mice were monitored for clinical signs and body weight changes over a 4-month period. At approximately 720, 1,080, 1,440, 2,160, and 2,880 h post-dose, terminal blood samples were collected and assayed for PF-05280014, trastuzumab-US, or trastuzumab-EU concentrations and anti-drug antibodies (ADA). Values for C max, area under the concentration time curve (AUC), clearance (CL), volume of distribution (V ss), half-life (t ½), and the presence of ADA were determined.
Results: In this report, peptide mapping of PF-05280014, trastuzumab-US, and trastuzumab-EU showed similar chromatographic profiles in a side-by-side analysis. The tumor cell growth inhibition of PF-05280014 was similar to trastuzumab-US and trastuzumab-EU. C max and AUC0-∞ values in mice were similar and dose-dependent across the mAbs at all doses, and CL and V ss values were similar and dose-independent. The CL values across doses ranged from 0.193 to 0.350 mL/h/kg (PF-05280014), from 0.200 to 0.346 mL/h/kg (trastuzumab-US), and from 0.193 to 0.335 mL/h/kg (trastuzumab-EU). V ss values across doses ranged from 84.9 to 120 mL/kg (PF-05280014), 86.7 to 130 mL/kg (trastuzumab-US), and 85.4 to 116 mL/kg (trastuzumab-EU). The incidence of ADA was low (~10%) and also similar across all dose levels and the three mAbs. The lower exposure generally observed in ADA-positive animals did not impact the overall PK interpretation. All animals survived to their scheduled terminal blood collection with no mAb-related differences in body weight gain or clinical signs.
Conclusions: PF-05280014, trastuzumab-US, and trastuzumab-EU were well tolerated during the 4-month observation period following a single dose of up to 100 mg/kg. PF-05280014, trastuzumab-US, and trastuzumab-EU showed similar structural properties, tumor cell growth inhibition properties, and PK profiles. The incidence of ADA was low and similar across the three mAbs. The results of these studies support the development of PF-05280014 as a proposed biosimilar to Herceptin.
Figures


References
-
- HERCEPTIN® [trastuzumab]: US prescription information. San Francisco, CA, USA: Genentech Inc.; 2010.
-
- European Medicines Agency. Herceptin: Authorisation. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici.... Accessed 30 Aug 2013.
-
- Committee for Medicinal Products for Human Use (CHMP). Guideline on Similar Biological Medicinal Products. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed 11 Mar 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

