MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer's disease
- PMID: 25001178
- PMCID: PMC4194100
- DOI: 10.15252/embj.201387576
MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer's disease
Abstract
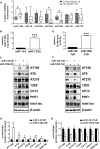
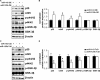
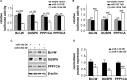
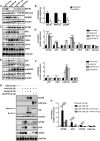
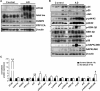
Sporadic Alzheimer's disease (AD) is the most prevalent form of dementia, but no clear disease-initiating mechanism is known. Aβ deposits and neuronal tangles composed of hyperphosphorylated tau are characteristic for AD. Here, we analyze the contribution of microRNA-125b (miR-125b), which is elevated in AD. In primary neurons, overexpression of miR-125b causes tau hyperphosphorylation and an upregulation of p35, cdk5, and p44/42-MAPK signaling. In parallel, the phosphatases DUSP6 and PPP1CA and the anti-apoptotic factor Bcl-W are downregulated as direct targets of miR-125b. Knockdown of these phosphatases induces tau hyperphosphorylation, and overexpression of PPP1CA and Bcl-W prevents miR-125b-induced tau phosphorylation, suggesting that they mediate the effects of miR-125b on tau. Conversely, suppression of miR-125b in neurons by tough decoys reduces tau phosphorylation and kinase expression/activity. Injecting miR-125b into the hippocampus of mice impairs associative learning and is accompanied by downregulation of Bcl-W, DUSP6, and PPP1CA, resulting in increased tau phosphorylation in vivo. Importantly, DUSP6 and PPP1CA are also reduced in AD brains. These data implicate miR-125b in the pathogenesis of AD by promoting pathological tau phosphorylation.
Keywords: Alzheimer's disease; kinases; microRNA‐125b; phosphatases; tau phosphorylation.
© 2014 The Authors.
Figures

References
-
- Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. - PubMed
-
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

