Binding mode characterization of NBD series CD4-mimetic HIV-1 entry inhibitors by X-ray structure and resistance study
- PMID: 25001301
- PMCID: PMC4135886
- DOI: 10.1128/AAC.03339-14
Binding mode characterization of NBD series CD4-mimetic HIV-1 entry inhibitors by X-ray structure and resistance study
Abstract
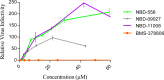
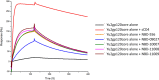
We previously identified two small-molecule CD4 mimetics--NBD-556 and NBD-557--and synthesized a series of NBD compounds that resulted in improved neutralization activity in a single-cycle HIV-1 infectivity assay. For the current investigation, we selected several of the most active compounds and assessed their antiviral activity on a panel of 53 reference HIV-1 Env pseudoviruses representing diverse clades of clinical isolates. The selected compounds inhibited tested clades with low-micromolar potencies. Mechanism studies indicated that they act as CD4 agonists, a potentially unfavorable therapeutic trait, in that they can bind to the gp120 envelope glycoprotein and initiate a similar physiological response as CD4. However, one of the compounds, NBD-09027, exhibited reduced agonist properties, in both functional and biophysical studies. To understand the binding mode of these inhibitors, we first generated HIV-1-resistant mutants, assessed their behavior with NBD compounds, and determined the X-ray structures of two inhibitors, NBD-09027 and NBD-10007, in complex with the HIV-1 gp120 core at ∼2-Å resolution. Both studies confirmed that the NBD compounds bind similarly to NBD-556 and NBD-557 by inserting their hydrophobic groups into the Phe43 cavity of gp120. The basic nitrogen of the piperidine ring is located in close proximity to D368 of gp120 but it does not form any H-bond or salt bridge, a likely explanation for their nonoptimal antagonist properties. The results reveal the structural and biological character of the NBD series of CD4 mimetics and identify ways to reduce their agonist properties and convert them to antagonists.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Singh IP, Chauthe SK. 2011. Small molecule HIV entry inhibitors. Part II. Attachment and fusion inhibitors: 2004–2010. Expert. Opin. Ther. Pat. 21:399–416 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

