Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD
- PMID: 25001321
- PMCID: PMC4251300
- DOI: 10.1136/bjophthalmol-2014-305252
Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD
Abstract
Background: Data comparing systemic exposure and systemic vascular endothelial growth factor (VEGF) suppression of ranibizumab, bevacizumab and aflibercept following intravitreal injection are lacking.
Methods: Fifty-six patients with wet age-related macular degeneration received intravitreal ranibizumab (0.5 mg), bevacizumab (1.25 mg), or aflibercept (2.0 mg). Serum pharmacokinetics and plasma free VEGF were evaluated after the first and third injections.
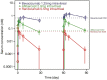
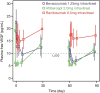
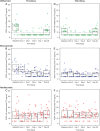
Results: Following the first dose, systemic exposure to aflibercept was 5-, 37-, and 9-fold higher than ranibizumab, whereas, bevacizumab was 9-, 310-, and 35-fold higher than ranibizumab, based on geometric mean ratio of peak and trough concentrations and area under the curve, respectively. The third dose showed accumulation of bevacizumab and aflibercept but not ranibizumab. Aflibercept substantially suppressed plasma free VEGF, with mean levels below lower limit of quantitation (10 pg/mL) as early as 3 h postdose until ≥7 days postdose. Mean free (unbound) VEGF levels with ranibizumab were largely unchanged, with mean trough level of 14.4 pg/mL compared with baseline of 17 pg/mL.
Conclusions: There are notable differences in systemic pharmacokinetics and pharmacodynamics among anti-VEGF treatments after intravitreal administration. All three agents rapidly moved into the bloodstream, but ranibizumab very quickly cleared, whereas bevacizumab and aflibercept demonstrated greater systemic exposure and produced a marked reduction in plasma free VEGF.
Trial registration number: NCT02118831.
Keywords: Retina.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- Rothen M, Jablon E, Monares G, et al. Anti-macular degeneration agents. Ophthalmol Clin North Am 2005;18:561–7. - PubMed
-
- Avastin (bevacizumab) solution for intravenous infusion prescribing information. http://www.gene.com/download/pdf/avastin_prescribing.pdf (accessed 11 Mar 2014).
-
- Lucentis (ranibizumab injection) intravitreal injection prescribing information. http://www.gene.com/download/pdf/lucentis_prescribing.pdf (accessed 11 Mar 2014).
-
- Xu L, Lu T, Tuomi L, et al. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci 2013;54:1616–24. - PubMed
-
- Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006;26:859–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
