Efg1 directly regulates ACE2 expression to mediate cross talk between the cAMP/PKA and RAM pathways during Candida albicans morphogenesis
- PMID: 25001410
- PMCID: PMC4187626
- DOI: 10.1128/EC.00148-14
Efg1 directly regulates ACE2 expression to mediate cross talk between the cAMP/PKA and RAM pathways during Candida albicans morphogenesis
Abstract
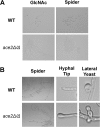
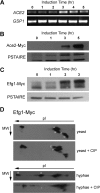
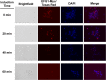
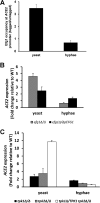
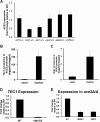
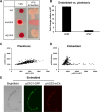
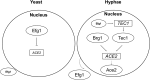
The cyclic AMP/protein kinase A (cAMP/PKA) and regulation of Ace2 and morphogenesis (RAM) pathways are important regulators of the yeast-to-hypha transition in Candida albicans that interact genetically during this process. To further understand this interaction, we have characterized the expression of ACE2 during morphogenesis. In normoxic, planktonic conditions, ACE2 expression is very low in stationary-phase cells at both the mRNA and protein levels. Upon shifting to Spider medium, ACE2/Ace2p levels increase. Although Ace2 is not absolutely required for hypha formation, ace2Δ/Δ mutants show delayed hypha formation in Spider medium (but not others) and morphological changes to the hyphal tip and lateral yeast. We also show that Efg1 directly binds the promoter of Ace2 in stationary phase, and ACE2 levels are increased in strains lacking Efg1 and the protein kinase A proteins Tpk1 and Tpk2, indicating that the PKA pathway directly regulates ACE2 expression. ACE2 expression is positively regulated by Tec1 and Brg1, which bind the promoters of ACE2 in hyphal cells but not in the yeast phase. Under embedded conditions, Efg1 is dispensable for filamentation and Ace2 is required. We have found that ACE2 expression is much higher in embedded cells than in planktonic cells, providing a potential rationale for this observation. Taken together, our observations indicate that the PKA pathway directly regulates the RAM pathway under specific conditions and are consistent with a model where the two pathways carry out similar functions that depend on the specific environmental context.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
The Ndr/LATS Kinase Cbk1 Regulates a Specific Subset of Ace2 Functions and Suppresses the Hypha-to-Yeast Transition in Candida albicans.mBio. 2020 Aug 18;11(4):e01900-20. doi: 10.1128/mBio.01900-20. mBio. 2020. PMID: 32817109 Free PMC article.
-
A large-scale complex haploinsufficiency-based genetic interaction screen in Candida albicans: analysis of the RAM network during morphogenesis.PLoS Genet. 2011 Apr;7(4):e1002058. doi: 10.1371/journal.pgen.1002058. PLoS Genet. 2011. PMID: 22103005 Free PMC article.
-
Morphogenesis-regulated localization of protein kinase A to genomic sites in Candida albicans.BMC Genomics. 2013 Dec 1;14(1):842. doi: 10.1186/1471-2164-14-842. BMC Genomics. 2013. PMID: 24289325 Free PMC article.
-
Transcriptional control of dimorphism in Candida albicans.Curr Opin Microbiol. 2001 Dec;4(6):728-35. doi: 10.1016/s1369-5274(01)00275-2. Curr Opin Microbiol. 2001. PMID: 11731326 Review.
-
Histone deacetylase-mediated morphological transition in Candida albicans.J Microbiol. 2015 Dec;53(12):805-11. doi: 10.1007/s12275-015-5488-3. Epub 2015 Dec 2. J Microbiol. 2015. PMID: 26626350 Review.
Cited by
-
Yeast casein kinase 2 governs morphology, biofilm formation, cell wall integrity, and host cell damage of Candida albicans.PLoS One. 2017 Nov 6;12(11):e0187721. doi: 10.1371/journal.pone.0187721. eCollection 2017. PLoS One. 2017. PMID: 29107946 Free PMC article.
-
The Cbk1-Ace2 axis guides Candida albicans from yeast to hyphae and back again.Curr Genet. 2021 Jun;67(3):461-469. doi: 10.1007/s00294-020-01152-1. Epub 2021 Jan 12. Curr Genet. 2021. PMID: 33433733 Free PMC article.
-
Systematic analysis of the Candida albicans kinome reveals environmentally contingent protein kinase-mediated regulation of filamentation and biofilm formation in vitro and in vivo.mBio. 2024 Aug 14;15(8):e0124924. doi: 10.1128/mbio.01249-24. Epub 2024 Jul 1. mBio. 2024. PMID: 38949302 Free PMC article.
-
Hbr1 Activates and Represses Hyphal Growth in Candida albicans and Regulates Fungal Morphogenesis under Embedded Conditions.PLoS One. 2015 Jun 3;10(6):e0126919. doi: 10.1371/journal.pone.0126919. eCollection 2015. PLoS One. 2015. PMID: 26039220 Free PMC article.
-
Intravital imaging-based genetic screen reveals the transcriptional network governing Candida albicans filamentation during mammalian infection.Elife. 2023 Feb 27;12:e85114. doi: 10.7554/eLife.85114. Elife. 2023. PMID: 36847358 Free PMC article.
References
-
- Moran GP, Coleman D, Sullivan D. 2012. An introduction to the medically important Candida species, p 11–25 In Calderone R, Clancy CJ. (ed), Candida and candidiasis, 2nd ed. ASM Press, Washington, DC
-
- Vazquez JA, Sobel JD. 2003. Candidiasis, p 143–187 In Dismukes WE, Pappas PG, Sobel JD. (ed), Clinical mycology. Oxford University Press, Oxford, United Kingdom
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

