An ahemolytic pneumolysin of Streptococcus pneumoniae manipulates human innate and CD4⁺ T-cell responses and reduces resistance to colonization in mice in a serotype-independent manner
- PMID: 25001458
- PMCID: PMC4271054
- DOI: 10.1093/infdis/jiu321
An ahemolytic pneumolysin of Streptococcus pneumoniae manipulates human innate and CD4⁺ T-cell responses and reduces resistance to colonization in mice in a serotype-independent manner
Abstract
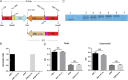
Background: Some Streptococcus pneumoniae serotypes express an ahemolytic pneumolysin (PLYa). Serotypes that commonly express PLYa, including serotype 8 (ST8) and ST1, are often associated with a low prevalence during colonization but a higher propensity to cause invasive disease. We sought to study the host response to ST8 PLYa in a homologous and heterologous capsular background.
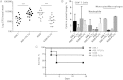
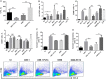
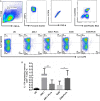
Methods: We genetically exchanged the PLYa of ST8 strain 6308 with the hemolytic PLY (PLYh) of ST3 A66.1 and vice versa and determined the impact of the exchange on nasopharyngeal colonization in mice. Then, to compare the response of human cells to PLYa-expressing and PLYh-expressing strains, we infected human peripheral blood mononuclear cells (PBMCs) with PLY-switched strains and assessed dendritic cell and CD4(+) T-cell responses by intracellular cytokine staining.
Result: Mice colonized with PLYa-expressing strains had significantly higher colonization densities than those colonized with PLYh-expressing strains, irrespective of capsular background. Compared with infection of PBMCs with PLYh-expressing strains, infection with PLYa-expressing strains induced diminished innate (dendritic cell cytokines, costimulatory receptor, and apoptotic) and adaptive (CD4(+) T-cell proliferative and memory interleukin 17A) responses.
Conclusion: Our findings demonstrate that PLYa has the potential to manipulate host immunity irrespective of capsule type. PLY exchange between STs expressing PLYa and PLYh could lead to unexpected colonization or invasion phenotypes.
Keywords: Streptococcus pneumoniae; T cell; apoptosis; colonization; dendritic cell; invasion; mice; pneumolysin; serotype.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Insights into the Evolutionary Relationships of LytA Autolysin and Ply Pneumolysin-Like Genes in Streptococcus pneumoniae and Related Streptococci.Genome Biol Evol. 2015 Sep 8;7(9):2747-61. doi: 10.1093/gbe/evv178. Genome Biol Evol. 2015. PMID: 26349755 Free PMC article.
-
Pneumolysin with low hemolytic activity confers an early growth advantage to Streptococcus pneumoniae in the blood.Infect Immun. 2011 Oct;79(10):4122-30. doi: 10.1128/IAI.05418-11. Epub 2011 Jul 25. Infect Immun. 2011. PMID: 21788389 Free PMC article.
-
Comparison of specific in-vitro virulence gene expression and innate host response in locally invasive vs colonizer strains of Streptococcus pneumoniae.Med Microbiol Immunol. 2021 Jun;210(2-3):111-120. doi: 10.1007/s00430-021-00701-w. Epub 2021 Mar 22. Med Microbiol Immunol. 2021. PMID: 33751214
-
What is different about serotype 1 pneumococci?Future Microbiol. 2012 Jan;7(1):33-46. doi: 10.2217/fmb.11.146. Future Microbiol. 2012. PMID: 22191445 Review.
-
[Research progress in pneumolysin].Wei Sheng Wu Xue Bao. 2017 Mar 4;57(3):333-40. Wei Sheng Wu Xue Bao. 2017. PMID: 29756432 Review. Chinese.
Cited by
-
Next generation protein based Streptococcus pneumoniae vaccines.Hum Vaccin Immunother. 2016;12(1):194-205. doi: 10.1080/21645515.2015.1052198. Hum Vaccin Immunother. 2016. PMID: 26539741 Free PMC article. Review.
-
Protection against Streptococcus pneumoniae Invasive Pathogenesis by a Protein-Based Vaccine Is Achieved by Suppression of Nasopharyngeal Bacterial Density during Influenza A Virus Coinfection.Infect Immun. 2017 Jan 26;85(2):e00530-16. doi: 10.1128/IAI.00530-16. Print 2017 Feb. Infect Immun. 2017. PMID: 27895132 Free PMC article.
-
Host-Pathogen Interactions in Gram-Positive Bacterial Pneumonia.Clin Microbiol Rev. 2019 May 29;32(3):e00107-18. doi: 10.1128/CMR.00107-18. Print 2019 Jun 19. Clin Microbiol Rev. 2019. PMID: 31142498 Free PMC article. Review.
-
Virulence factors of Streptococcus anginosus - a molecular perspective.Front Microbiol. 2022 Oct 26;13:1025136. doi: 10.3389/fmicb.2022.1025136. eCollection 2022. Front Microbiol. 2022. PMID: 36386673 Free PMC article. Review.
-
Genome-wide identification of lineage and locus specific variation associated with pneumococcal carriage duration.Elife. 2017 Jul 25;6:e26255. doi: 10.7554/eLife.26255. Elife. 2017. PMID: 28742023 Free PMC article.
References
-
- Richards L, Ferreira DM, Miyaji EN, Andrew PW, Kadioglu A. The immunising effect of pneumococcal nasopharyngeal colonisation; protection against future colonisation and fatal invasive disease. Immunobiology. 2010;215:251–63. - PubMed
-
- Witzenrath M, Pache F, Lorenz D, et al. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J Immunol. 2011;187:434–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

